For me, cancer is one of the most fascinating topics in medicine because a lot of evolutionary and biological principles come together.
Cancer detection and treatment have seen little progress over the last decades – particularly considering the vast resources that have been thrown at it. I now present three avenues I believe will revolutionize cancer treatment. If all of these will come to bear fruit, nobody of us will have to die of cancer.
Cancer in general, and the strategies I personally follow aiming to prevent it, are discussed here.
Liquid biopsies
The earlier cancer is detected, the exponentially better it can be treated. Very early cancer that has not yet started to be locally invasive can simply be excised. Conversely, after cancer has formed distant metastases it is a death spell 100% of the time (with the exception of melanoma).
The best cancer prevention strategy is simply early detection. This is where liquid biopsies come in handy.
A “biopsy” is a procedure that involves taking a small sample of body tissue and then examining it more closely. With a “liquid” biopsy, the tissue to be sampled is the blood (therefore the term “liquid”). In principle, it looks like this.
- First, blood is taken.
- Then, all free-swimming DNA is amplified via PCR.
- Thereafter, the DNA is examined more closely to potentially detect whether there might be some DNA that stems from cancer cells. This is done via algorithms developed by machine learning approaches.
- Lastly, the results give a prediction about what bodily tissues are likely to have or have not cancer. The negative predictive value is particularly strong. For example, the liquid biopsy results can tell you with a near 100% certainty that you do not have e.g., lung or pancreatic cancer.
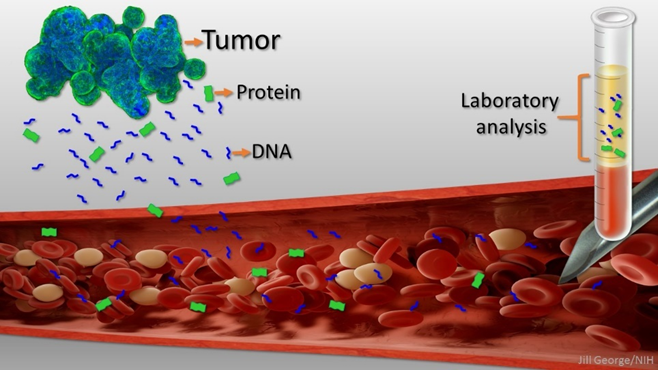
If liquid biopsies end up working out the way researchers hope, a liquid biopsy could predict with reasonable accuracy how likely it is that a patient has a certain type of cancer. Practically, this means that I would take a blood test and the results would tell me what kind of cancer (or cancers) I could have. The organs that result in a positive (e.g., “It is 16% likely that you have a small lung cell carcinoma.”) are then examined in more detail by conventional medical screening technologies.
Given there is no major war or global catastrophe, liquid biopsies may be the standard of care in a decade or so. Given that early detection is the most efficient and effective way to go about preventing cancer, it would be hard to overstate how this might revolutionize cancer treatment. Whether liquid biopsies can live up to their expectations remains to be seen.
Changing the principles of how we do chemotherapy
Cancers are incredibly “smart” evolutionary machines. Currently in oncology, a tumor is treated with the maximum tolerated dose of a combination of chemotherapeutic drugs, and the drug cocktail is only changed if the cancer comes back (i.e., it has grown resistant).
In contrast, it would (likely) be better to rapidly alternate treatments, so that cancer cells never build true resistance (because a specific resistance mechanism is expensive to build and maintain). This would mean, getting the cancer cells to very small numbers using treatment #1 and then hitting these cells again with treatment #2, and so on and so forth. (Instead of always giving treatment #1 only.)
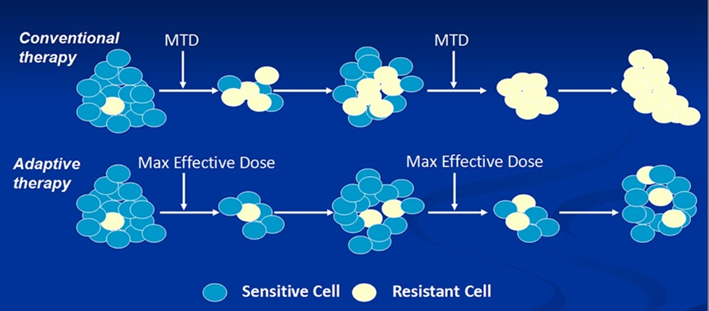
Extinction would be achieved through a rapid alternating sequence of perturbations, none of which by itself would cause extinction. As cancer researcher Robert Gatenby says: “Over the last few decades, we have been looking for a magic bullet, but maybe all we need is a series of pretty good bullets.” And we have a lot of pretty good bullets already.
Developing and perfecting immunotherapy
Immunotherapy for cancer treatment is based on the concept that tumor cells have mutations that distinguish them from normal cells. This, in theory, makes it possible for our own immune system to recognize these “foreign” cells and then kill them. In fact, our immune system is taking out thousands of (pre)cancerous cells per day, completely outside of our awareness.
A cancer cell usually has between 70-150 mutations. Of the roughly 100 mutations per cancer, around 1% of these mutations (about 1 mutation per patient) fit into a patient’s HLA molecules and are therefore immunogenic (meaning that the immune system can detect it as foreign).
In fact, it is believed that the immune system of about 80% of patients recognizes at least one antigen from their tumor. Unfortunately, in many instances, tumor cells evolved to inhibit the immune system (e.g., via employing PD1 & CTLA4 pathways) and/or dampen the immune response (e.g., by secreting immunosuppressive cytokines such as IL-10 or TGF-beta).
A class of drugs called “checkpoint inhibitors” is already being used to block the tumor’s dampening of the immune response (and therefore to dis-inhibit the patient’s own immune system). However, these drugs are only effective against highly mutated cancers (e.g., cancers harboring 1000 mutations or more such as melanoma), but they have little impact on typical cancers (i.e., cancers with a normal mutation count of 50-150 mutations). Furthermore, they often lead to autoimmune attacks on healthy tissues.
The fact that there is one or more tumor-specific antigens per cancer can be exploited more selectively by unleashing a patient’s own immune system to target this antigen. This can be achieved via (at least) two different ways:
- Adoptive T-cell transfer: First, the set of T-cells capable of attacking the tumor is isolated. Then, they are expanded ex vivo (i.e., they are multiplied in a petri dish). Then, they are infused back into the patient. This tumor-specific T-cell army then attacks the tumor.
- CART cell therapy: The binding region of a soluble antibody that is capable of targeting a tumor-specific protein is fused to the T-cell receptor, which then works like a membrane-bound “antibody” to find and take out tumor cells. These assassin cells are then expanded ex vivo and given back to the patient, attacking the tumor.
The difference between these approaches is that with adoptive T-cell transfer the patient’s own lymphocytes are used, whereas with CART technology the cells are genetically engineered.
The upside of immunotherapy is that it is 100% selective (and thus the closest thing to a “magic bullet”) with little to no “off-target”-killing (vs. surgery, radiation, or chemotherapy – which all harm non-cancerous tissue as well).
In the future, immunotherapy may be able to cure most cancers. The major drawback is that immunotherapy can hardly be scaled. Tumor antigens are rarely shared between patients and thus immunotherapy needs to be developed for each patient individually, making immunotherapy hard to create on a mass scale.
An alternative to immunotherapy is the concept of “oncolytic viruses”, of which there are many kinds and variations. In principle, we let viruses do what they do best: infect and kill cells. Oncolytic viruses are designed in a way that they infect tumor cells (and ideally only tumor cells) and kill them.
CART, adoptive cell therapy, and oncolytic viruses are all at various stages of development and are likely available within the next decade or two.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Sources & further information
- Podcast: Peter Attia & Max Diehn: Liquid biopsies and cancer detection
- Podcast: Peter Attia & Robert Gatenby: Viewing cancer through an evolutionary lens and why this offers a radically different approach to treatment
- Podcast: Peter Attia & Steven Rosenberg: The development of cancer immunotherapy and its promise for treating advanced cancers
Disclaimer
The content available on this website is based on the author’s individual research, opinions, and personal experiences. It is intended solely for informational and entertainment purposes and does not constitute medical advice. The author does not endorse the use of supplements, pharmaceutical drugs, or hormones without the direct oversight of a qualified physician. People should never disregard professional medical advice or delay in seeking it because of something they have read on the internet.
