Many individuals possess a limited or overly basic understanding of neurotransmitters. Yet, when leveraged effectively, such knowledge can be immensely powerful. In this article, I provide a comprehensive summary of neurotransmitter science and explore various methods for their modulation.
Serotonin (“calmness”)
Serotonin was named after its ability to constrict blood vessels (“sero” as in serum and “tonin” as in tone). About 90% of the human body’s total serotonin is located in the GI tract, 8% in platelets, and 2% in the CNS.
I will only discuss serotonin’s role in the CNS, and not the rest of the body, where serotonin serves a variety of functions such as regulation of vasoconstriction, platelet aggregation, and gastrointestinal balance, among other things.
However, even in the CNS, serotonin is by far the most complex of the monoamines because is implicated in a large number of different functions. In Homo sapiens, there are 11 different (relevant) serotonin receptors, all but one of which are coupled to G-proteins (Gs; Gi; Gq). I will discuss G-protein coupled receptors in more detail shortly. The distribution and density of these receptors vary in different areas of the brain.
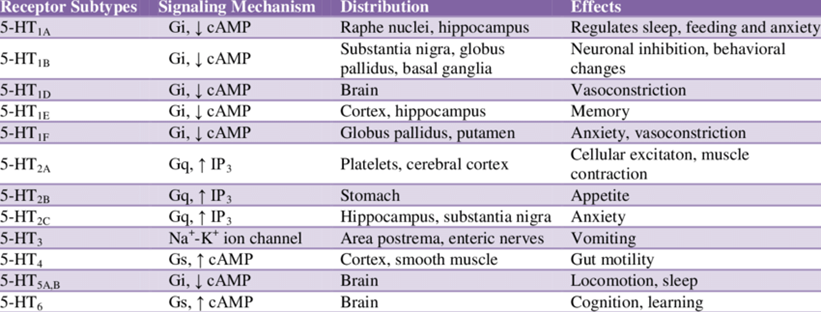
In the human brain, there are only about 200.000 serotonin-producing neurons, in some people more and in some people less. Most of these are located within the raphe nuclei in the brain stem, but their projections extend to many areas of the brain and spinal cord.
Whereas noradrenaline and dopamine serve a couple of quite specific functions, this is not the case for serotonin.
- Noradrenaline is responsible for the execution of sympathetic nervous system functions and an increase in CNS activity and arousal.
- Dopamine is responsible for incentive salience (motivation), focus, movement, and inhibition of prolactin.
- Serotonin participates in a myriad of different functions.
The functions of serotonin include:
- inhibition of pathways associated with fear (including the inhibition of the fight-or-flight response)
- modulating basal ganglia systems
- thermoregulation
- sleep induction
- pain modulation
- perception
- affecting hypothalamic neuroendocrine control (e.g., regulating the HPA-axis, regulating the release of oxytocin, etc.)
- mediating the feeling of satiety
- regulating sex drive
- modulating the release of catecholamines
In contrast to dopamine and noradrenaline, many of these serotonergic functions are independent of each other. That is, there are specific subpopulations of serotonergic neurons that are primarily implicated in regulating one specific function.
The serotonin function recognized by the wider public is “calmness”. This calmness is in part a result of the inhibition of pathways associated with fear (most of the serotonin receptors are inhibitory receptors). The inhibition of neural networks involved in the fear response, thereby reducing “background” fear, increases calmness.
Serotonin also plays a crucial role in shaping one’s self-perception and confidence. One theory suggests that individuals with lower serotonin levels may view themselves as being at the bottom of the social hierarchy, a concept known as the “neurobiochemistry of defeat”. Conversely, elevating serotonin levels through SSRIs is hypothesized to potentially overturn power dynamics in social or romantic relationships. On SSRIs, in general, people tend to have higher self-worth and self-confidence. One friend who uses an SSRI to combat Long COVID-induced anxiety coincidentally found that the SSRI made him a much tougher negotiator.
Serotonin is also implicated in regulating cognitive flexibility, which is thought to be in part by its modulation of basal ganglia systems. When people who are “locked” into rigid mental and behavioral patterns are given SSRIs or other serotonergic drugs, the thought loops, rumination, and cognitive rigidity often lessen. Therefore, serotonergic drugs are often prescribed for OCD, depression, and eating disorders – and other disorders characterized by excessive cognitive rigidity. After ruminating about various things for years, a friend of mine found that a very low dose (1.25mg) of escitalopram effectively reduces his tendency to ruminate – no psychotherapy needed, simply elevating his serotonin by a small amount was enough.
Low levels of serotonin are also associated with poor agreeableness, irrational coping mechanisms, impulsivity, overanalyzing, and poor ability to just “be present”.
Under physiological conditions, serotonin signaling in one region is independent of serotonin signaling in any other region. However, with pharmacological intervention, namely getting a molecule into the bloodstream that perturbs molecular signaling in a certain way, all of these functions are modulated in one go.
For example, SSRIs block the SERT protein throughout the body – and not just where we want them to. SSRIs block SERT-transporters not just in “fear centers” but also in appetite centers, sleep centers, the gastrointestinal system, the heart, and all of the other places where SERT transporters are found. This explains why SSRIs have such a wide variety of effects and side effects. SSRIs are discussed in more detail here: Thoughts on SSRIs
Serotonin is to a large extent metabolized by the enzyme MAO-A into the inactive metabolite 5-HIAA. Some studies have successfully correlated violence, aggression, suicide, reduced social status, and some types of depression with low concentrations of cerebrospinal fluid 5-HIAA.
Conversely and paradoxically, when serotonin levels get extremely high (serotonin syndrome), the most common symptom is not excessive calmness but rather agitation and aggression. Furthermore, loss of appetite, tremors, hyperreflexia, and hyperthermia often occur.
Once, as an experiment, I took a low dose of 5HTP (a precursor to serotonin) sublingually while I was on moclobemide, which elevated serotonin in a “natural” way (unlike SSRIs, which change synaptic cleft amplitude differences and therefore elevate serotonin in an unnatural way). For about half an hour I felt what “pure” serotonin feels like – a warm fuzzy feeling of intense calmness, contentment, and gratitude, somewhat comparable to a small dose of MDMA.
Noradrenaline (“alertness”)
Noradrenaline is one of the major excitatory transmitters in the human brain. “Excitatory” means that it increases neuronal activity. The locus coeruleus – the brain’s major source of noradrenaline – is located in the brain stem and from there, noradrenergic neurons project to the whole nervous system.
In contrast to most other neurotransmitters, the noradrenaline system lacks specificity. Whenever activity in the locus coeruleus is upregulated, noradrenaline is “sprinkled” over the whole brain.
In the context of noradrenaline, I will briefly discuss G-protein-coupled-receptor signaling, which is crucial to understanding neurotransmitter action. The following paragraphs may be a little technical for laypeople.
Whenever a neuron comes in contact with a monoamine neurotransmitter (such as noradrenaline, dopamine, serotonin, or histamine), the monoamine attaches to specific transmembrane receptors of a receptor family called G-protein-coupled-receptors (GPCR), which then activate a specific cellular pathway. There are millions of these receptors per neuron.
Each GPCR is then coupled to one of three pathways “instructing” a cell to do one of three things:
- The Gs pathway (“G” stands for GPCR; “s” stands for stimulatory) activates a specific protein called protein kinase A, which phosphorylates a large number of downstream targets, basically instructing a cell to do more of what it is already doing (“Dear cell, please do more of what you are already doing.”).
- The Gi pathway (“i” stands for inhibitory) deactivates protein kinase A, which downregulates cellular activity (“Dear cell, please do less of what you are already doing.”)
- The Gq pathway (“q” stands for q-subunit) elevates intracellular calcium and activates protein kinase C, both of which have a wide range of cellular effects, depending on the protein machinery of the target cell.
In the case of noradrenaline, there are five different receptors, also called “adrenergic” receptors. Three of these receptors are coupled to the Gs-pathway (the so-called beta-adrenergic receptors), one to the Gq-pathway (the alpha1 adrenergic receptor), and one to the Gi-pathway (the alpha2 adrenergic receptor).
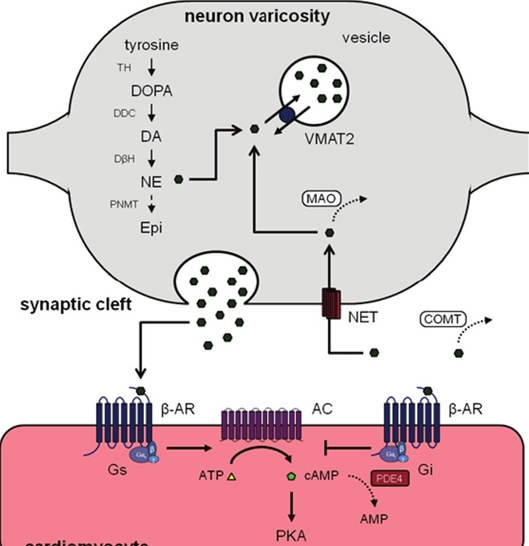
Different receptor subtypes are expressed by different types of cells in a variety of bodily tissues. For example, the alpha-1 adrenergic receptor is widely expressed by arterioles throughout the body leading to vasoconstriction, and the beta-1 adrenergic receptor is expressed by various cardiac cells (heart cells) leading to a greater workload (e.g., faster heartbeat, greater contractile force, faster signal conduction, etc.).
Almost every neuron in the brain carries hundreds of thousands of adrenergic receptors. Much of the noradrenaline in the brain acts on the Gs-pathway (“Dear cell, please do more of what you are already doing.”), which then instructs cells to upregulate activity. Therefore, increased noradrenergic activity usually means an increase in overall central nervous system activity, among other things. (In reality, things are much more complex, but I try to keep it simple.)
Therefore, simplistically speaking, everything that raises noradrenaline levels amounts to an increased activity of the whole nervous system. Among other things, this results in greater alertness, cognition, focus, perception, and stronger emotions.
For example, when people are anxious or excited, noradrenergic transmission is generally high, and blocking it can reduce anxiety or excitement. For this reason, beta-blockers (blocking beta-adrenergic receptors) are often used to combat stage fright, and I used them occasionally myself before giving a presentation. Interestingly, after having done this a couple of times (5-10mg of propranolol an hour before giving a talk), I did not need it anymore because I learned that I could effectively give a presentation without getting anxious – so, for me and friends who used this hack, there was no “psychological” dependence, quite the opposite.
Most stimulants increase noradrenaline levels either directly (e.g., amphetamine) or indirectly (e.g., modafinil, caffeine). Other things that increase noradrenergic tone are a variety of hormones, being cold, stress, excitement, exercise, or fasting.

Noradrenaline action is terminated by the reuptake of noradrenaline through the noradrenaline transporter (NET), or by metabolism (breakdown) of noradrenaline by monoamine oxidase A (MAO-A) and COMT. Therefore, noradrenaline levels can be increased by NET inhibition (e.g., methylphenidate, reboxetine, bupropion), or MAO inhibition (e.g., moclobemide).
Noradrenaline release can also be stimulated by the activation of trace-amine-associated-receptor 1 (TAAR1), for example by dexamphetamine.
Dopamine (“motivation & cognition”)
Dopamine is critical to approach behaviors of all kinds and to the capacity to switch from one behavior to another. Dopamine is one of the primary (biological) determinants of how excited someone is, how motivated, and how ready one is to push through things to get what he wants.
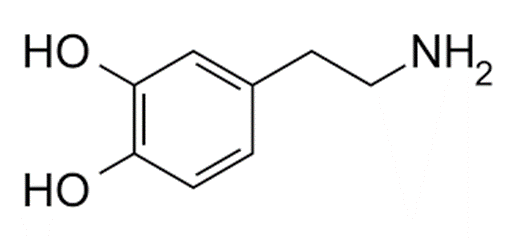
There is a widespread misconception that dopamine is the “pleasure neurotransmitter”. It is not. Instead, dopamine has mostly to do with incentive salience (a fancy term for “motivation”).

In scientific terms, incentive salience is called “wanting”, whereas pleasure is called “liking”. Pleasure (“liking”) is mostly mediated by opioidergic pathways in the nucleus accumbens shell. Opioid systems are discussed in more detail shortly.
The vertebrate reward system 101
The “wanting” + “liking” pathways form the endogenous reward system. To simplify, the release of dopamine initially regulates the “wanting” sensation (e.g., desiring chocolate). Upon fulfilling the desired target (e.g., consuming chocolate), endorphins (which are naturally occurring opiates) then induce the “liking” sensation.
Example. If one experimentally blocks dopamine in rats, they starve to death because they would not do any work to get to the food. However, if food is put into their mouth, their pleasure reaction is unchanged (as measured by orofacial muscle activation).

Conversely, if one blocks opioidergic pathways, they will still work to acquire food, but they do not “like” eating it.
Dopamine signaling
There are five different dopamine receptors (D1- D5) coupled to two different functional intracellular signaling pathways, Gs and Gi. In general, dopamine release activates a certain set of brain networks implicated in motivation, cognition, and motor behavior.
It does so either directly by being coupled to neurons expressing Gs-coupled pathways (“Dear cell, please do more of what you are already doing.”), or it does so by activating Gi-coupled pathways on inhibitory interneurons (“Dear cell, please do less of what you are already doing.”).
In the latter case, dopamine signaling then functions as an “off-switch” to these inhibitory interneurons, leading to disinhibition, which in the process upregulates the net output of a certain network. The famous D2 receptor is of this type, the major target of antipsychotic drugs.
At its core, dopamine works similarly to noradrenaline. Namely, increasing or decreasing the activity of certain neural networks. However, while noradrenaline is distributed to the whole brain and spinal cord, dopamine is much more selective in its targets.
There are four major dopamine pathways.
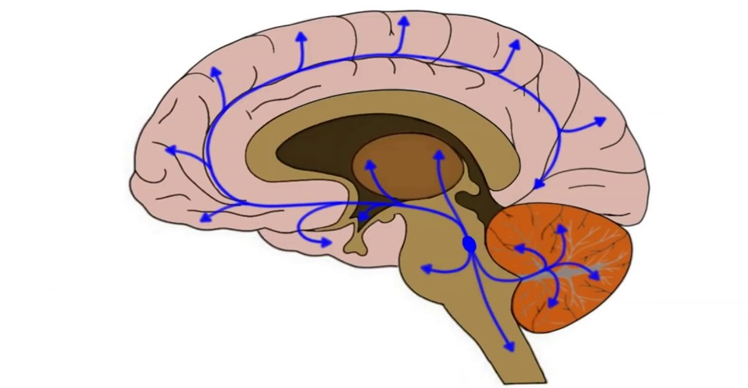
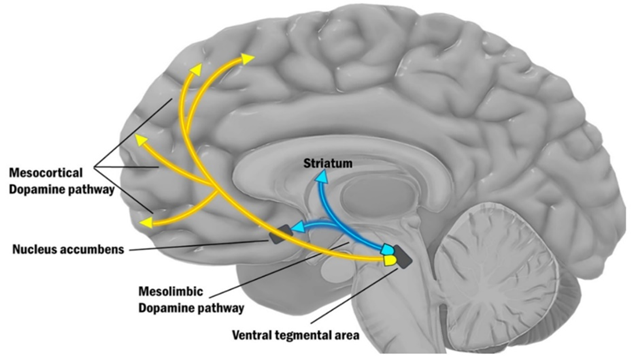
- The mesolimbic pathway (“motivation”): As the name implies, the mesolimbic pathway projects from the midbrain (“meso”) to the limbic system (“limbic”), more specifically the nucleus accumbens. The mesolimbic pathway is important for regulating motivation and approach behavior (“wanting”). This pathway is part of the reward system, as explained above.
- The mesocortical pathway (“cognition”): This pathway projects from the midbrain (“meso”) to the prefrontal cortices (“cortical”). The mesocortical pathway is important for regulating focus & cognition. In this pathway, expression of the dopamine transporter (DAT) is low, and most of the dopamine reuptake is carried out by the noradrenaline transporter NET. For this reason, noradrenaline reuptake inhibitors such as reboxetine are a viable treatment for ADHD because they increase domain levels in the prefrontal cortex. I share my thoughts on ADHD here: ADHD – To Treat Or Not to Treat?
- The nigrostriatal pathway (“movement”): In this pathway, dopamine projects from the substantia nigra (SN) to the dorsal striatum. The nigro-striatal pathway is important for regulating motor behavior (movement). This pathway is most famous for being dysfunctional in Parkinson’s disease (though in reality, the other dopamine pathways are just as dysfunctional).
- The tuberoinfundibular pathway (“prolactin”): In this pathway, dopamine controls the release of prolactin. Therefore, dopamine agonists are clinically used to treat high prolactin levels.
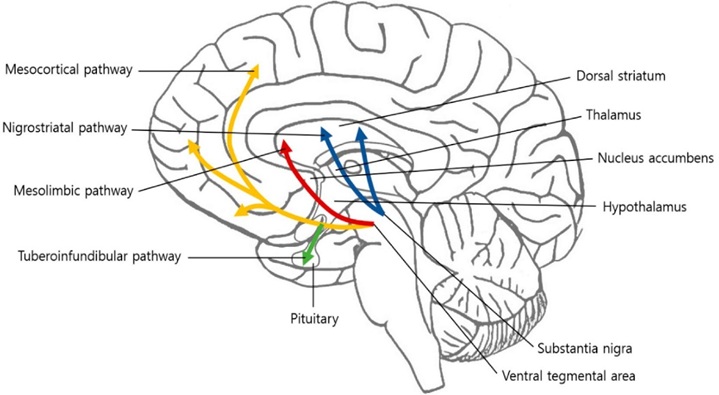
For our purposes, we only care about the first two pathways, the mesolimbic pathway (shown above as the red arrow) and the mesocortical (yellow arrows).
The mesolimbic pathway is important for motivation.
In the mesolimbic pathway, a collection of dopamine neurons in the ventral tegmental area (VTA) projects to the nucleus accumbens, which is the entry point into the limbic loop of the basal ganglia system.
The mesolimbic dopamine system mediates incentive salience (motivation), which is critical to vitality, curiosity, agency, libido, an ability to anticipate reward (to look forward to things), and a sense of urgency and importance.
Hyperdopaminergic states tend to increase the range of activities an organism finds worthwhile pursuing. Conversely, hypodopaminergic states result in anhedonia, loss of motivation, and a lack of perceived meaning in one’s life experience.
This pathway is thought to be dysfunctional in ADHD, some forms of depression, and anhedonia. The brutal neglect of dopamine in psychiatry is discussed here.
There are a variety of things that are thought to decrease dopamine levels in this pathway. Important among these are obesity, low testosterone, low cortisol, some kinds of depression, and any form of addiction. On the pharmaceutical side there are simulants such as modafinil or amphetamine.
Most stimulants temporarily increase activity in the mesolimbic pathway, and therefore they tend to increase one’s motivation to act. However, they are rarely viable long-term solutions (as discussed here).
The mesocortical pathway is important for focus and cognition.
In this pathway, a collection of dopamine neurons in the VTA projects to various key populations of neurons in the frontal cortices, amygdalae, anterior cingulate cortices, and hippocampi.
There, dopamine upregulates pathways that improve focus (the narrowing of attention) and cognition (executive functions), which is useful for helping with goal-directed behavior (e.g., planning).
Usually, the mesolimbic and the mesocortical pathways operate together. That is, dopamine release in these two pathways happens at the same time.
- The mesolimbic pathway mediates the “wanting” to do something specific. For example, “I want to eat chocolate”.
- Meanwhile, dopamine released through the mesocortical pathway activates brain networks associated with executive functions, such as improving focus and sharpening working memory. These improvements in cognition then help with attaining the target of the “wanting” (i.e., cognition is harnessed to execute the correct behaviors to attain the chocolate).
Therefore, whenever dopamine levels rise, motivation (mesolimbic pathway) and cognition (mesocortical pathway) increase at the same time. In fact, dopaminergic stimulants do not only improve motivation to act, but they also improve mental performance – at least temporarily.
Interestingly, dopamine transporter (DAT) expression is low in the prefrontal cortices, and most of the dopamine reuptake in the mesocortical pathway happens via the noradrenaline transporter (NET). Therefore, noradrenaline reuptake inhibitors, such as reboxetine, improve dopamine transmission selectively in the mesocortical system.
Dopamine, personality, and aging
As I was using selegiline, a selective MAO-B inhibitor elevating dopamine levels, I found that it made me more restless, impulsive, curious, and agentic. While I was incredibly productive on it, I also found that it changed my personality in not-so-favorable ways. I discuss my experience with selegiline in more detail here.
As mentioned in the section on guiding principles, biological factors ripple through someone’s life in the same way an Earthquake ripples through the Earth’s crust.

Different dopamine receptor polymorphisms are correlated with certain aspects of human personality. For example, a specific genotype of the dopamine D4 receptor is correlated to the personality trait of “novelty-seeking”, which is characterized by exploration, risk-taking, curiosity, and impulsiveness.
Furthermore, personality changes with aging. While there are many reasons, I believe that a reduction in dopaminergic tone is not to be neglected.
Compared to adults, children appear hypomanic at baseline. They are emotional, have great energy, are curious, and act impulsively. This is thought to be in part due to their high dopaminergic tone. As they get older, they get less curious and enthusiastic. This may not be exclusively due to them being tortured by archaic education systems, but may also be in part because dopamine levels progressively decline.
In fact, of the about 85 billion or so neurons, only a mere 400-500 thousand neurons manufacture dopamine (the actual count varies between individuals), and approximately 5-10% of these neurons are lost per decade.
Furthermore, testosterone and cortisol levels also decrease with aging, which are two hormones that significantly regulate dopaminergic tone.
I believe that next to Groundhog Day setting in, this progressive reduction in dopaminergic drive is part of the reason people who were once brimming with energy, ambition, and a sense of purpose in their twenties, often transition from productive go-getters to couch potatoes.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Histamine (“wakefulness”)
Histamine is a monoamine neurotransmitter but it is not as well-known as other monoamines (noradrenaline, serotonin, and dopamine). This is likely because it is not commonly used for therapeutic purposes because its activation can trigger the release of histamine from mast cells throughout the body resulting in vasodilation and itching.
Next to its role in the brain, histamine is also involved in the inflammatory cascade and can activate a variety of leukocytes, making it an important transmitter in the immune response.
In the brain, histamine is primarily important for maintaining wakefulness. Histamine is released from the tuberomammillary nucleus of the hypothalamus and projects widely throughout the brain. Much of it is coupled to the Gq-pathway.
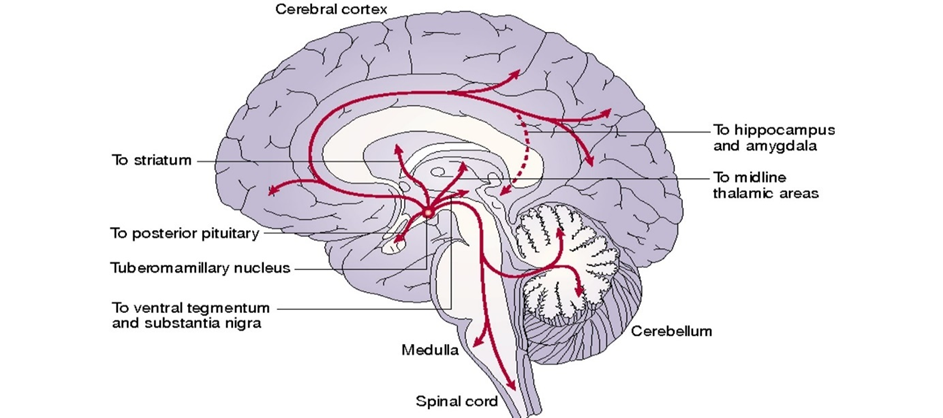
While directly activating the histamine system is not a smart idea because of its involvement in inflammation, antihistamine drugs are commonly employed as sleep agents for their drowsiness-causing properties (e.g., trazodone, mirtazapine, quetiapine, Nyquil).
Recently, a drug called pitolisant, an H3 receptor antagonist has been approved for narcolepsy. By antagonizing inhibitory autoreceptors, pitolisant increases brain histamine levels, thereby stimulating wakefulness. Another drug that is well known for indirectly increasing central histamine levels is modafinil.
Histamine is tightly coupled to the orexin system, both activating each other. The orexin system is important for “stabilizing” monoaminergic systems, particularly the histamine system. Orexin levels strongly rise with fasting and being hungry (which is one of the reasons people cannot fall asleep when hungry). The orexin system is dysfunctional in narcolepsy (sufferers are lethargic all around the clock and fall asleep suddenly when emotionally aroused).
Increased orexin levels are one of the major reasons why mutations in the DEC2 gene (the “short sleeper gene”) allow people to sleep for only 5 hours per night.
Glutamate (“activity”)
While noradrenaline and histamine are the major long-range and long-acting excitatory transmitters, glutamate is the major short-range and fast-acting excitatory neurotransmitter.
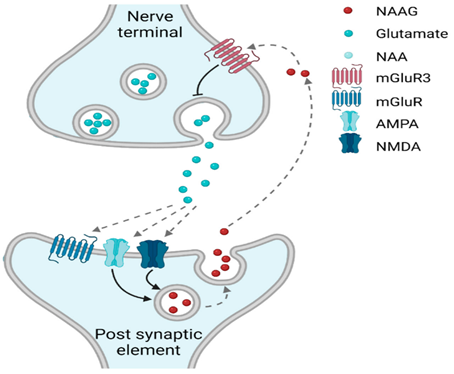
Glutamate is crucial to vitality, energy levels, and proper CNS activity. Whenever glutamate signaling increases, so does information transmission.
In contrast to monoamine transmitters, glutamate regulation happens more locally. Nonetheless, baseline glutamate levels rise whenever levels of noradrenaline, histamine, or cortisol rise.
Glutamate activates ion channels of the AMPA and NMDA-receptor class. (Next to ion channels, there are also glutamatergic G-protein-coupled receptors, but they are beyond the scope of this article). I will briefly discuss both:
- AMPA receptors are crucial to short-range excitation. Most of the glutamate receptors are of this type. They are basically the counterpart to GABA-A receptors, which are crucial to short-range inhibition.
- The NMDA receptor is crucial to long-term potentiation (LTP), which strengthens a specific connection.
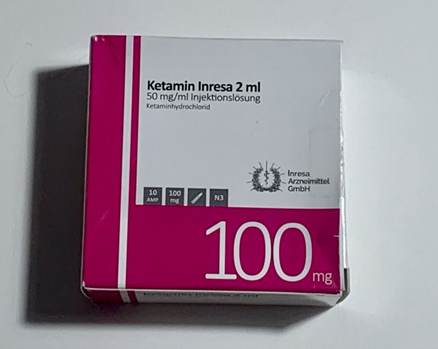
Here, I want to mention ketamine. Ketamine blocks the NMDA receptor, which leads to a rebound upregulation of glutamate signaling through the AMPA receptor, which then produces a range of neurotrophic responses thought to be responsible for the efficacy of ketamine as an antidepressant. I discuss my experience with ketamine in more detail here.
I also want to mention ampakines, which are positive allosteric modulators of the AMPA receptor. Ampakines are to the AMPA receptor what benzodiazepines are to the GABA A receptor (discussed next). Research on ampakines may potentially deliver powerful nootropics in the not-so-distant future. The major downside is that they are potentially neurotoxic (“excitotoxicity”). Ampakines are discussed in more detail in the article on piracetam.
GABA (“calmness”)
Whereas glutamate is the major short-acting excitatory neurotransmitter, GABA is the major short-acting inhibitory neurotransmitter. About 90% of cortical neurons are primarily GABAergic.
GABA does the opposite of glutamate, namely reducing the likelihood that a neuron fires. Increasing GABAergic tone reduces CNS activation, which results in a calming, sedating, relaxing, amnesic, and sleep-promoting effect.
There are two classes of GABA receptors:
- GABA-A receptor: Most of the GABA receptors are GABA-A receptors, which are chloride ion channels. When this receptor is activated, the influx of chloride ions hyperpolarizes a neuron and therefore suppresses neuronal excitation. This receptor is the target of benzodiazepines, which increase the likelihood that this receptor is activated. (To make things more complex, there are a variety of different GABA-A receptor subunits with somewhat different functional involvement. However, discussing them is beyond the scope of this article.)
- GABA-B receptor: These are not ion channels, but rather G-protein-coupled receptors linked to the Gi-pathway (“Dear neuron, do less of what you are currently doing.”). Baclofen and phenibut act on this receptor.
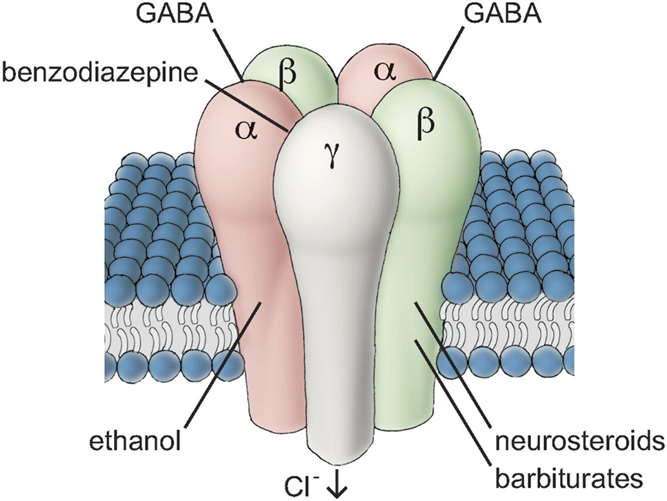
GABAergic drug use is associated with tolerance, counterregulation, and short-term cognitive dysfunction.
Long-term administration of GABAergic molecules also leads to cognitive impairment and raises the risk for dementia (longer-acting drugs more than shorter-acting drugs). GABAergic drugs include benzodiazepines, Z-drugs, gabapentinoids, alcohol, and phenibut. I discuss many of them in the article on sleep drugs.
GABAergic drugs are commonly employed as anti-anxiety agents, tranquilizers, and hypnotic drugs.
Acetylcholine (“attention & memory”)
There are various cholinergic transmitter systems in the human brain. In general, acetylcholine is sort of an “activity-amplifier”.
One important cholinergic nucleus is the nucleus basalis (NB) in the forebrain. This nucleus supplies a variety of cortical areas with acetylcholine, amplifying their activity.
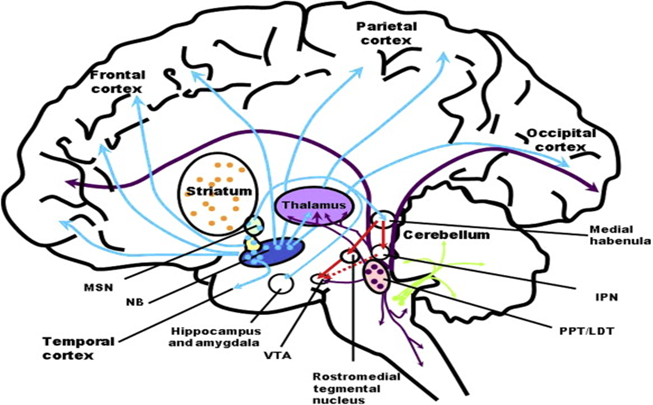
Drugs that block cholinergic receptors, such as antipsychotics or tricyclics, are essentially “dumb drugs” and they are also associated with cognitive impairment and dementia. Conversely, drugs that increase levels of acetylcholine, such as cholinesterase inhibitors, are often employed for the symptomatic treatment of dementia, and “off-label” to induce lucid dreaming.
Nicotine, an agonist at several nicotinic receptors, is a potent nootropic, and I use it frequently. I discuss my experience with nicotine here.
The opioid system (“pleasure”)
There are multiple different independent opioid systems distributed throughout many tissues of the human body, in which the expression of opioid receptors serves to transmit inhibitory signals for a variety of functions. All opioid receptors are coupled to the Gi-pathway of GPCR signaling.
For example:
- mediating the feeling of reward (“pure pleasure”) in the nucleus accumbens (hence their recreational abuse is described as “kissing God”)
- inhibiting pain transmission (hence their use as pain medications)
- regulating gastrointestinal motility (hence the constipation for opioid users)
- hypothalamic signaling (hence the decrease in testosterone with opioid addiction)
- regulating breathing (hence the respiratory depression that often ends deadly)
- the cough reflex (hence the use of codeine as the prototypical antitussive agent)
- regulating the immune system (hence the use of low dose naltrexone as an immunomodulator)
- regulating a variety of brain functions
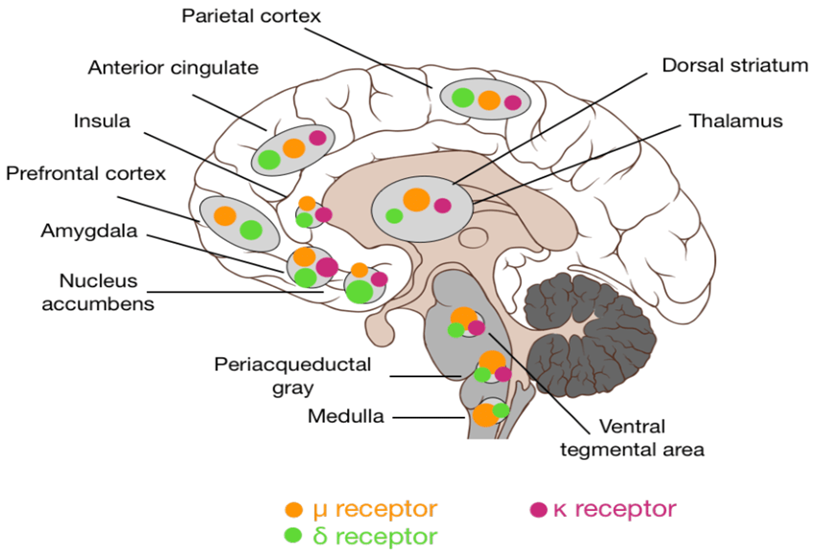
I will limit my discussion to the involvement of opioids in the “pleasure” response.
It evolved that endogenous opioids are released into the nucleus accumbens shell whenever a vertebrate animal (fish; amphibians; reptiles; birds; mammals) is engaging in an activity that had been proven to be evolutionarily adaptive, such as sex, sweetness, or bathing in the sun. These activities have evolved to be pleasurable because they have been increasing reproductive success for millions of years.
The signature of the pleasurable feeling itself (”pure pleasure”) is mostly a release of endogenous opioids into the brain’s major hedonic hotspot – the nucleus accumbens shell. The function and purpose of this opioid release is to signal “Hey animal, keep doing whatever you are doing.”
The major receptor involved is the infamous mu-opioid receptor. Over 100 polymorphisms are known in the mu-opioid receptor gene.
Selective mu-opioid agonists, such as endorphin, heroin, oxycodone, or morphine, typically induce euphoria, whereas selective kappa agonists, such as dynorphin, typically induce dysphoria. A cruel negative feedback loop exists between the kappa- and mu-opioid systems. If the kappa system is activated (e.g., by strenuous exercise) the mu-opioid system is upregulated, and vice versa. Partially for this reason does exercise make people feel good for hours after (an increase in other monoamines does contribute).
Mu-agonists produce euphoria/pleasure and kappa-agonists produce dysphoria/malasie. A synthetic kappa-receptor antagonist with the name aticaprant is in Phase III development for MDD. Unfortunately, in former trials, they tended to cause heart issues.
The acute effects of mu-opioid agonists can seem magical. Users feel well – sometimes for the first time in their lives. However, with prolonged use, insidious counterregulatory mechanisms kick in.
Among other things, the baseline mu-opioid signaling downregulates, and the kappa-opioid system upregulates. Hence, users feel dysphoric whenever they do not use opioids. Addicts say that simply wanting to relieve the negative state is as much a motivation to keep going as is chasing the high. The well-known cycle of addiction starts and often ends deadly. In the process, exogenous opioid users cause immense suffering to themselves, their friends and family, and society at large.

I believe that it is good that these drugs are banned (or at the very least highly regulated) almost everywhere on Earth as they hijack the major hedonic hotspot (nucleus accumbens shell) within the brain’s reward system in the most direct manner possible.
Nonetheless, weaker opioidergic drugs such as tianeptine (a weak mu-receptor agonist), buprenorphine (a mu-partial agonist and a kappa-antagonist), or tramadol (a weak mu-receptor agonist, and an SNRI) can be useful for select cases of treatment-resistant depression – at least for a short period of time until desensitization and counterregulation set in.
However, using opioidergic drugs (“pure pleasure”) therapeutically in depressed individuals has considerable risks and downsides, particularly their addictiveness. I discuss the potential usefulness of opioids in the treatment of depression, as well as the opioid crisis, in more detail in my article on tianeptine: My Experience With Tianeptine.
Therefore, when it comes to the treatment of depression, the opioid system has been left mostly untouched -even more so than the dopaminergic system, which is the other neglected transmitter system important to well-being (and not just the elimination of misery). The brutal neglect of dopamine is discussed here.
An often-abused indirect opioidergic drug is the cannabinoid THC, which is discussed here.
Of note, as an experiment, I temporarily blocked my opioid receptors with naltrexone, which has taught me that a life without endogenous opioid signaling is barely worth living. I discuss my experience in more detail here.
Recently, ACKR3, a chemokine receptor that functions as a scavenger for opioids has been identified. Inhibiting this receptor would elevate synaptic opioid levels in an analogous way an SSRI elevates synaptic serotonin levels. Inhibitors are currently in clinical trials for opioid use disorder.
A brief history of humanity’s fondness for opioids is discussed here.
Pharmacology 101
Normally, signaling systems are mainly activated locally (perhaps except for noradrenaline and histamine). However, when a molecule makes its way into the bloodstream, for example by oral administration, it comes in touch with all cells. It then acts on all of the cells that express receptors for which the molecule has an affinity. These receptors are then activated, partially activated, or inactivated – depending on the effect of the molecule on a certain receptor.
For example, if caffeine makes it into the bloodstream, it is distributed via the circulatory system to every cell of the body. Caffeine then acts on all of the trillions of adenosine receptors, more technically the A1, A2A, A2B, and A3 receptors, throughout the body. It inactivates some of these and partially activates others.
Caffeine does not just act where we want it to act – namely, in the ventrolateral preoptic area in the hypothalamus and the dopamine neurons in the ventral tegmentum. Caffeine also acts on blood vessels, kidneys, the heart, and many other tissues.
Similarly, if somebody injects diacetylmorphine (heroin), the mu-opioid receptor is not just activated in the nucleus accumbens shell (the brain region where “pure pleasure” originates from) but all of the other mu-opioid receptors throughout the body are activated as well.
And if somebody takes an SSRI, the SERT transporter is blocked all over the body. This includes the SERT transporters in appetite centers, sleep centers, fear centers, the gastrointestinal system, the heart, and all of the other places where SERT transporters are found.
Under physiological conditions, opioid, adenosine, or serotonin signaling in one region are completely independent of opioid, adenosine, or serotonin signaling in any other region. But when a molecule is put into the bloodstream that has an affinity for these receptors, all of these formerly independent regions are activated in one go. This is part of the reason most pharmaceutical drugs have a number of side effects.
What determines the setpoint of these neurotransmitters?
Let’s look at someone with bipolar disorder. During their “up” phase, they feel great, have drive, experience high libido, and are a charge of energy. This “up”-period is in part brought about by various neurotransmitters being set to a high baseline state.
Gradually the switch into the “down” phase begins. They start to feel “like shit”, are depressed, and have abysmal energy levels. The major thing that changed between “up” and “down” are the levels and signaling of a variety of neurotransmitters (presumably as a consequence of a change in the expression of a number of genes).

Every one of us has a certain “setpoint” for the various levels of neurotransmitters, which determine a non-minor part of our energy, mood, motivation, emotionality, etc. at any given time. The baseline levels of these transmitters are influenced by many things.
- Genetics & early life: Setpoints are partly due to genetics, embryonic development, and early life upbringing. Together, these will determine the number, synaptic connections, and baseline activity of neurons responsible for synthesizing and releasing the individual neurotransmitters.
- Age: Neurotransmitters are generally at their peak during childhood and adolescence and gradually decrease thereafter. This is due to the myriad of different mechanisms comprising the phenomenon we call “aging”. Most individuals are quite vital – even hypomanic – as little children. They have boundless energy, are motivated, curious, enthusiastic, capable of feeling profound feelings, and often have lofty dreams and goals. As individuals age, most lose vitality gradually but progressively and most individuals are a little less vital during adolescence, even less during adulthood, and have little vitality in old age.
- Hormones: Hormones have a major say in neurotransmitter levels. These include insulin, leptin, thyroid hormones, and steroid hormones such as vitamin D, cortisol, estradiol, and testosterone. I discuss hormones in detail here.
- Mindset: It is thought that “mindset” can influence neurotransmitter levels top-down in an analogous way neurotransmitter levels can influence mindset bottom-up. For example, having a pessimistic or nihilistic mindset can sustainably alter monoamine transmission and hormones negatively (which then in turn favors a nihilistic or angst-ridden mindset). I discuss the (biological) importance of mindset in more detail here.
- Cyclical variations: Every one of us is subject to cyclical variations in neurotransmitter function. Bipolar disorder is just the most extreme end of the spectrum. Cycles can be quite erratic and cycle lengths can range from weeks to months. For many people, cycles are not intense enough to notice. These cyclical variations are the primary reason why many people feel a little worse during winter. Cyclical variations can be brought about by changes in sunlight, seasons, lifestyle, life circumstances (e.g., falling in love, grief, finding purpose, peak experiences, etc.), or simply by poorly understood genetic oscillations.
Furthermore, many other factors can modulate neurotransmitter levels. These include sleep, diet, exercise, body fat, and immune activation such as allergies, autoimmune diseases, or infections, among others. The influence of cytokines on brain function is discussed here.
Sources & further information
- Podcast: Andrew Huberman: Understanding & conquering depression
- Textbook snippet: Monoamine Hypotheses of Mood Disorders
- Scientific review: Neurobiology of Attention Deficit/Hyperactivity Disorder
Disclaimer
The content available on this website is based on the author’s individual research, opinions, and personal experiences. It is intended solely for informational and entertainment purposes and does not constitute medical advice. The author does not endorse the use of supplements, pharmaceutical drugs, or hormones without the direct oversight of a qualified physician. People should never disregard professional medical advice or delay in seeking it because of something they have read on the internet.
