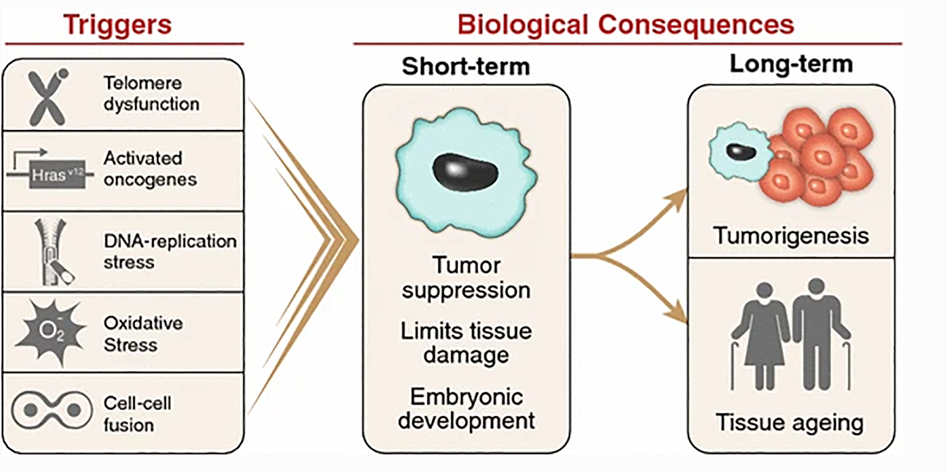
In the pathology of aging, it seems that many roads lead to the formation of senescent cells, which are possibly a key feature of aging. With each cell division, telomeres shorten and cells move progressively closer to the Hayflick limit (for human fibroblasts about 75 divisions), after which they stop dividing and become senescent.
Other upstream pathways leading to cellular senescence include elevated mTOR signaling, or cellular catastrophes such as ER stress, mitochondrial damage, and widespread DNA damage. Downstream pathways include a long list of age-related diseases.
Cellular senescence is a state of irreversible cell-cycle arrest. Senescent cells, also dubbed “zombie cells”, are dysfunctional cells that are not well capable of performing their functions properly but occupy valuable tissue space. Furthermore, they secrete a nasty cocktail of pro-inflammatory mediators called SASP (senescence-associated secretory phenotype). Some researchers also believe that senescent cells induce senescence in neighboring cells, initiating a vicious positive feedback loop.
Often, the transition to senescence is driven by the activation of a protein called p53, but there are other ways to induce senescence. Regardless, senescent cells change gene expression in ways that are (mostly) unfavorable to proper tissue health.
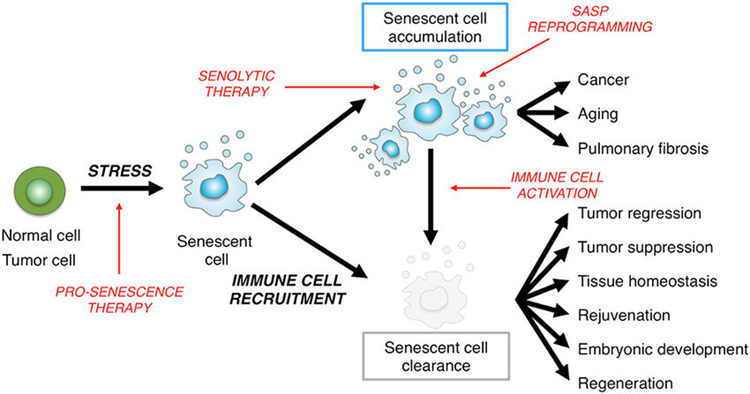
The ability to make senescent cells is vitally important for survival. One function of the cellular senescent system is to be an anti-cancer system. If a mouse is genetically engineered to be incapable of making senescent cells, it will be full of tumors quite early in life.
However, if senescent cells are eliminated after they are already senescent, it is suspected that this may have a number of rejuvenating effects on the organism spanning multiple aspects of anatomy and physiology.
Once again, the unintended consequences of cellular senescence represent a system that was awesome for the young reproductively competent organism at the expense of the old.
As animals age, these “zombie cells” accumulate, in the process impairing tissue health and function via two mechanisms:
- Senescent cells are dysfunctional cells that do not perform their job as well as healthy cells. Because they do not die, they unnecessarily occupy vacant niches, preventing stem cells and neighboring cells from repopulating these niches with healthy functional cells.
- Senescent cells are thought to create a damaging bystander effect as they secrete a host of “shitty” mediators (senescence-associated secretory phenotype – SASP) of pro-inflammatory cytokines, myokines, and proteases that impair tissue maintenance and function, and perhaps even accelerate the advent of senescence for surrounding healthy cells. Broadly speaking, most of these mediators are implicated in causing inflammation. In total, over a hundred of these mediators have been characterized, though SASP identity differs between tissues.
For example, in a primate study it was found that the number of senescent skin fibroblasts increase non-linearly with age. At 5 years about 1% of skin fibroblasts were senescent, at age 15 that number increased to 5%, at age 25 to 15%, and at 30 years of age (which is very old for most primates) about 30-35% of skin fibroblasts were senescent.
Fortunately, senescent cells are highly dependent on continuous signaling from certain growth factors and growth pathways, which can be targeted pharmacologically, eventually driving senescent cells toward apoptosis.
Senolytics
Senolytics are molecules that preferentially kill senescent cells. Senolytics do not mess with the ability to create senescent cells (which may promote tumor growth), but clear out cells that are already senescent. The use of senolytics makes (theoretical) biological sense for a variety of conditions as senescent cells have been implicated in the pathogenesis of a large variety of conditions.
In animal models, clearing senescent cells has been shown to ameliorate a plethora of age-related pathologies including atherosclerosis, osteoporosis, osteoarthritis, sarcopenia & frailty, hearing loss, hepatic steatosis (fatty liver), cataract formation, obesity-induced metabolic dysfunction, and possibly neurodegeneration.
Out of about 60 or so molecules screened, dasatinib (a receptor tyrosine kinase inhibitor used to treat leukemias) was the most potent “general purpose” senolytic agent tested, possibly being effective against a number of tissues. Furthermore, dasatinib has been in use for about two decades and there is lots of safety data and clinical experience to draw from.
In multiple long-term mouse studies, there were no apparent harmful long-term effects from the intermittent removal of senescent cells.
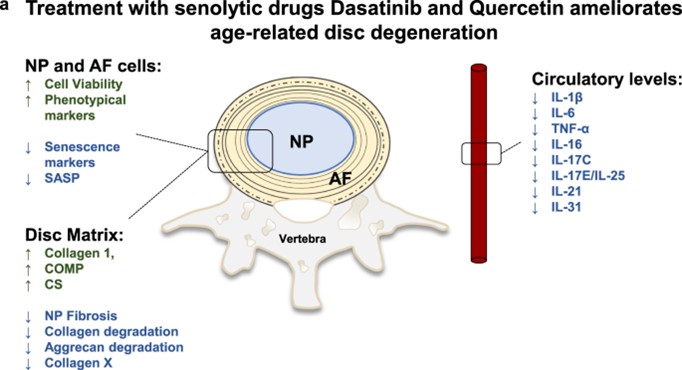
In one popular study, D&Q was started intermittently in very old mice (the human equivalent of about 80 years). There was a reported 36% increase in lifespan calculated from the time D+Q was started (about a 6% median extension of life span).
In another study, 20-month-old mice were treated with dasatinib + quercetin (D&Q) once monthly for four months and it was found that repeated D&Q administration prevented age-related bone loss, including improved bone microarchitecture, increased cortical thickness and bone strength, and a reduced number of senescent osteocytes.
Similar studies found analogous changes in a number of other organs.
Different tissues have senescent cells that rely on different growth factors and their receptors. Unfortunately, dasatinib only targets some tissues while others are left untouched. For example, dasatinib is highly effective against senescent skin fibroblasts or adipocytes but it is less effective against endothelial cells or fibroblasts.
Nonetheless, “hit-and-run” treatment with senolytic agents, which in the case of D + Q have elimination half-lives of about 10 hours, is sufficient to decrease senescent cell burden in multiple tissues for many weeks (in humans presumably for many months).
Other senolytics worth mentioning are the macrolide antibiotics azithromycin and roxithromycin. Both of these antibiotics have been used for decades in the treatment of cystic fibrosis and it was poorly understood why they were so effective and more effective than other antibiotics. It turns out that these two macrolide antibiotics selectively kill off senescent fibroblasts. Interestingly, rapamycin, doxycycline, roxithromycin, or dasatinib are ineffective for this.
As longevity MD Alan Green, who has been treating patients with rapamycin and senolytics for years, says: “You go to war with what you have; not what you want. This is what we have at this point in time.”
In the coming years, other senolytics capable of targeting other tissues will likely become clinically available. In fact, a handful of new senolytic drugs are currently in various stages of clinical development, including MDM2 inhibitors and BCL2 inhibitors, both of which inhibit the inhibitors of apoptosis. For example, if a MDM2 inhibitor is injected into a human osteoarthritic knee, joint pain is much reduced – sometimes for many months.
Other experimental senolytics target S6K, PI3K, and MEK, all of which are implicated in senescence-promoting networks.
Furthermore, some researchers are currently working on identifying biomarkers for senescent cells (such as β -galactosidase activity and the cell-cycle inhibitors p16INK4a and p21CIP1), which are then used to properly program CART cells to specifically target and eliminate clear senescent cells. Thus, if things go well, it may be possible to program CART cells to not just eliminate cancerous cells but also to employ them as an anti-aging therapy.
Personal experience with senolytics
The above is only a fraction of the article. This article is currently undergoing final revisions and is expected to be published within the next few weeks to months. To receive a notification upon its release, sign up for my newsletter.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
