mTOR inhibitors are the only pharmacological agents that extend lifespan across all eucaryotic organisms they were tested on. In fact, mTOR is the only pharmacological target that consistently shows anti-aging effects if it is inhibited.
Simplistically speaking, when mTOR is highly active, cells are in “growth mode”, and they are in “conserve-resources mode” when the mTOR pathway is poorly active (complete mTOR inactivity equals death).
mTOR is a protein that is highly conserved throughout all domains of eucaryotic life (protists, fungi, plants, animals). When there are sufficient nutrients & energy (glucose, leucine, arginine, methionine) and sufficient extracellular growth signals (e.g., insulin, IGF-1, tissue-specific growth factors), the mTOR complex is activated and drives a host of anabolic processes, including growth and development.
Because animals usually die long before aging kills them, evolution never bothered with reducing the activity of the mTOR pathway after sexual maturity had been reached. Thus, to the detriment of the old animal, mTOR is set at too high a level after growth and development have been completed. mTOR inhibitors are a potential chemical fix for the problem evolution never bothered to address.
Reducing the activity of the mTOR pathway combats many aspects of the aging process. It improves metabolic health and helps prevent atherosclerosis, cancer, and dementia. Furthermore, in laboratory animals, mTOR inhibition slows down the rate of degeneration of the brain, heart, bones, kidney, liver, skin, adrenal glands, ovaries, and stem cells.
Some researchers believe that mTOR inhibitors are the most geroprotective molecules currently available to mankind. While there are over 90 rapalogs known, only two are available for clinical use, including sirolimus (rapamycin) and its closely related cousin everolimus.
Rapamycin & aging
Rapamycin, like penicillin, was the product of biological warfare between bacteria and yeast. Certain bacteria evolved to synthesize mTOR inhibitors to inhibit the growth of yeast. It was found in the soil of Easter Island (Rapa Nui – thus also the name) in the 1960s.
It has long been postulated that the mTOR pathway is among the most promising pathways associated with aging. In experiments it was then found that mTOR inhibition is indeed a viable way to delay some aspects of age-related deterioration and a large body of basic science studies have shown that increased activity of mTOR is at the center of age-related disease.
For example, inhibiting the mTOR pathway with rapamycin was found to slow the rate of accumulation of senescent cells, sharpen the adaptive immune system, improve cellular and tissue health, reduce the risk of cancer, delay the onset of neurodegeneration, and improve metabolic health. Furthermore, rapamycin seems to be pretty good at reducing age-related sterile inflammation.
Applied topically, rapamycin can reduce (and even reverse) some aspects of skin aging.
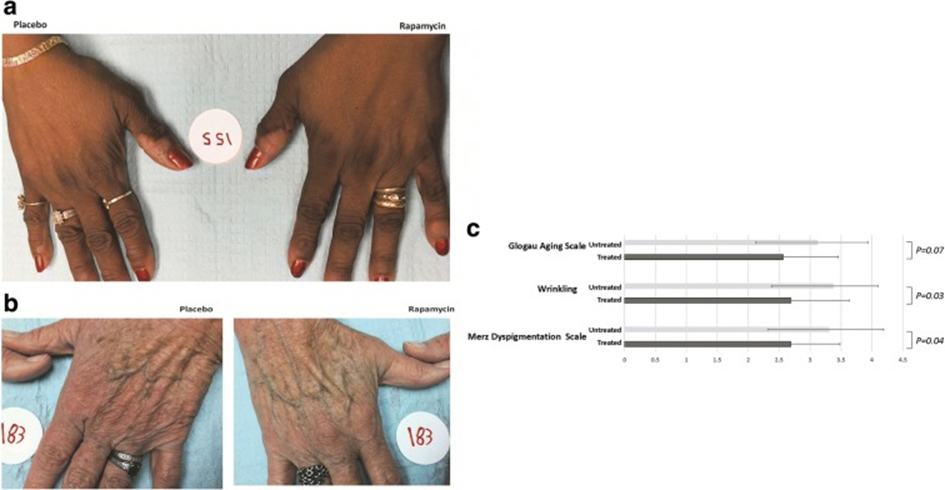
Essentially, rapamycin is suspected (and hoped) to delay some aspects of aging itself. Furthermore, if mTOR is inhibited in old animals, the consequent activation of stem cells might even result in some true rejuvenation (though true rejuvenation is probably slight). However, the primary power of mTOR inhibitors lies presumably in slowing down the aging process but not reversing it.
Clinically, rapamycin is classified as an “immunosuppressant”, however, some researchers hypothesize that treatment with rapamycin may restore immune function in an aged animal instead of suppressing it, speculatively through enhanced stem cell function, specifically if it is given in an intermittent rather than a continuous fashion.
For general health and longevity, it is presumably best to cycle periods of growth and autophagy. Intermittent dosing of rapamycin (e.g., a single weekly dose) might achieve this pharmacologically without the need for varying caloric intake (such as with multi-day fasting or caloric restriction).
However, the jury is still out on whether it is better to take low doses of mTOR inhibitors continuously (e.g., 1mg of rapamycin per day) versus higher doses intermittently (e.g., 7mg of rapamycin once weekly).
A couple of longevity doctors speculate that a single weekly dose (e.g., 4-8mg once per week) represents a good tradeoff between safety and efficacy, though there is currently little human data to support this (however, the absence of evidence is not evidence of absence).
In the Intervention Testing Program (ITP), the most rigorous anti-aging research in mice, rapamycin potently prolonged the lifespan (and health span) of both male as well as female mice, though it seems to work a little better in females, presumably because of increased absorption.
When giving mice rapamycin, they euthanized them at 22 months (most of them were still alive and fairly healthy). After death, they looked at dozens of different organs as well as their tendons. Their tendons were youthful. Their kidneys, heart, endometrium, liver, and adrenal glands did not have the “aged” characteristics of the control mice.
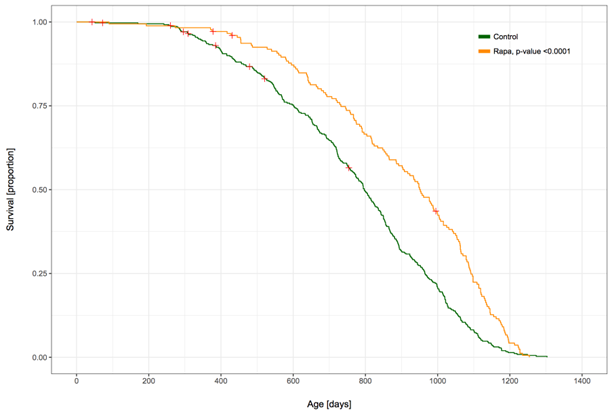
In the ITP, rapamycin increases the lifespan of male mice by 15%, by itself and by 29% if combined with acarbose. It is hard to overstate what a gain this represents. For example, if all cancers & heart attacks & strokes were eliminated today, the median increase in human lifespan would be only about 10%.
Will rapamycin increase human health span or even lifespan? Biological first principles & animal experiments answer this question with a tentative yes. However, nobody knows whether this is actually true. The major thing I am personally worried about is its potential adverse effect on neuroplasticity.
Personal experience with rapamycin
Back in 2017, I stumbled upon a podcast featuring Dr. Peter Attia, in which he discussed something about as unknown as himself was at the time – rapamycin. Intrigued, I delved into existing literature on the subject. It’s a leap of faith that whatever works in mice and model organisms also works for humans. However, it seemed to me that the risks were quite low given the data we have on rapamycin.
The best I can get is additional dog data. Unfortunately, the dog aging project will take a couple more years to complete. Given my risk appetite, I soon decided to take it.
I started with 1mg per week and titrated up by 1mg every other week. The first couple of weeks, I did have some annoying canker sores, which I also got whenever I took rapamycin after an occasional multi-day fast.
During the first 1-2 months, my blood sugar was elevated. More specifically, my fasting blood sugar went up from 90mg/dl to about 100-130mg/dl. Interestingly, my blood ketones were also quite high at the same time (though back then I was on a paleo-ketogenic diet). During the first two months, I also noticed a slight pitting edema on both of my legs, which may have been associated with rapamycin’s inhibitory effect on lymphatic vessel formation.
Because the side effects were mild and transient, I slowly titrated up to 10mg per week and stayed there for about one year (which I now realize was perhaps too high a dose). I get extensive blood work done once per quarter, and throughout this time all the blood markers remained mostly unchanged, other than borderline thrombocytopenia at the beginning.
During my time on 10mg/week, I did have one potentially severe adverse effect. At the time of starting rapamycin, I was doing heavy sprints on concrete about twice per week, and a couple of months after starting rapamycin I developed some knee pain in both of my knees. Even though an MRI showed no signs of pathology, it may have been possible that rapamycin impaired my healing abilities, resulting in pain.
The knee pain did not get better until I took a 3-month break from rapamycin, after which the pain went away completely and has not come back to this day. It is impossible for me to say what the cause was, but it is conceivable that the high doses of rapamycin did play a role. After this 3-month break, I restarted rapamycin at a lower dosage of 5mg per week.
Despite these side effects, I feel that, for me, taking rapamycin once per week is likely safe – over the long run perhaps even safer than not taking it. However, I might be wrong in this regard – there are quite a few unknowns.

Some people worry that rapamycin inhibits muscle growth. Since starting rapamycin, I did gain about 5-6kg of lean muscle, though I also started doing CrossFit during this time. It seems that, at least for me, muscle growth was not noticeably impaired.
People sometimes comment on how young I look, though it is hard to tell whether this is due to rapamycin, my other interventions, my lifestyle, or simply genetics.
I do extensive blood work 4-6 times per year and pretty much every time everything looks good. CRP is rarely measurable, homocysteine is very low, ApoB is below reference range (I do not take lipid-lowering drugs), fasting triglycerides about 30-50mg/dl, ALT/AST usually below 25 U/L, hormone markers are decent with my IGF-1 being a little high, and my IGF-1 is a little above reference range. My insulin sensitivity is great as well, as discussed here.
However, most of the changes related to rapamycin happen presumably deep within cells and cannot be measured through a blood test.
Unfortunately, I am not a wizard capable of peering into alternate dimensions to judge how I would do without the rapamycin. Thus whether the rapamycin did anything beneficial is hard to say. Though, at least for me, rapamycin did not have any noticeable adverse effects during the four years I have been taking it, with the possible exception of an exacerbation in knee pain.
I recently switched from sirolimus (rapamycin) to everolimus 5mg once weekly. My reasons are two-fold. Firstly, everolimus has a much shorter half-life (30 vs. 60h). Secondly, for me, everolimus is cheaper. Furthermore, everolimus seems to be a little more apt at crossing the blood-brain barrier, which though I am not sure is a good thing (could be good, could be bad).
There also seem to be indicators that, compared to sirolimus (rapamycin), everolimus is a little more selective for the mTOR1 complex vs. the mTOR2 complex, with potentially reduced metabolic side effects. However, the reduced side effects are probably just due to the much shorter half life.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Some technical stuff
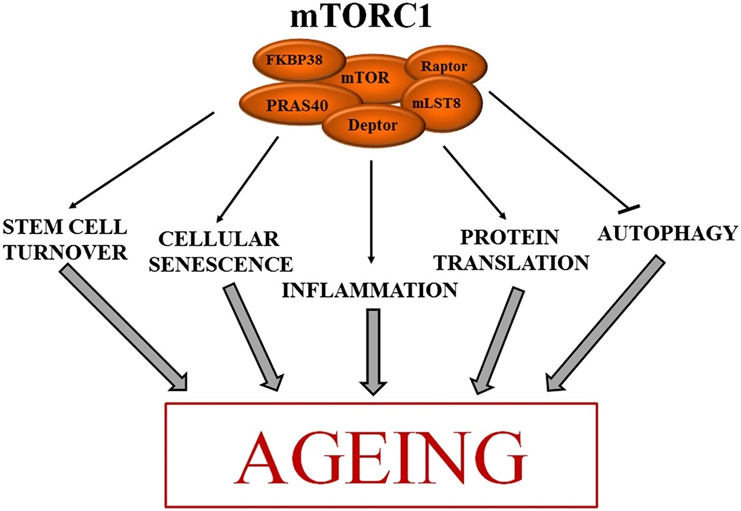
mTOR is a central hub in the intracellular micro-universe of the cell and one of the main regulators of cellular growth, cell size, and differentiation. mTOR is a kinase. A kinase is a protein that phosphorylates (adding a phosphate group) other proteins, which results in a conformational change leading to altered activity of the target protein – usually either increased or decreased activity.
Each kinase has one or more target proteins (also called “substrates”). In the case of mTOR, the number of target proteins presumably numbers in the dozens. The substrates of mTOR are involved in a variety of processes, including cell growth, cell proliferation, cell motility, cell survival, protein synthesis, autophagy, and transcription.
mTOR itself is regulated by many different intracellular and extracellular signals, including the availability of nutrients (e.g. ATP, glucose), amino acids (particularly leucine and methionine), and growth factors (e.g. IGF1, insulin, tissue-specific growth factors).
Likewise, mTOR activity is downregulated when nutrients are depleted (e.g., fasting), protein intake is restricted (e.g., vegan diet), or growth factors are lacking (e.g., low-carb diet). The downregulation of mTOR activity then causes many proteins needed for growth to be turned off, while the activity of many proteins involved with autophagy and repair are turned on.
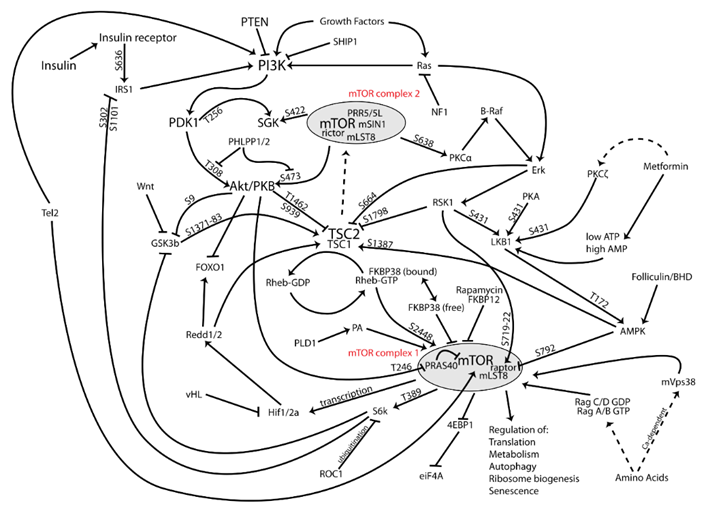
There are two main forms of mTOR. mTOR complex I and mTOR complex II (shown in red in the image above). In general, it is thought that the inhibition of complex I is associated with the things we want (e.g. autophagy induction, counteraction of growth pathways, reduction in cellular growth and proliferation, prevention of senescence), while many unwanted side effects are thought to come from the inhibition of complex II (e.g., a worsening of glucose homeostasis, adverse effects on the lipid profile, immunosuppression).
However, the notion that mTOR1 inhibition is “good” and mTOR2 inhibition is “bad” is probably bullshit and it is likely that the degree of mTOR1 inhibition itself is responsible for the side effects that were wrongly attributed to mTOR2.
Rapamycin is an mTOR inhibitor that is a little more selective for the mTOR complex I than complex II. More technically, rapamycin binds to FKBP12 (and a little bit to FKB52), and the FKBP12-rapamycin complex then binds to the mTOR1 complex, which modulates the ability of mTOR to phosphorylate a variety of downstream proteins.
For example, rapamycin-bound mTOR potently inactivates a protein called S6 kinase, which is important for mediating protein synthesis, and it also weakly binds to a protein called ULK1, which is an early step in the activation of the cell recycling machinery (autophagy). However, what substrates are responsible for rapamycin’s ability to delay aging is currently unknown.
So, in theory, rapamycin is a potent suppressor of protein synthesis and a less potent activator of autophagy. To make things even more complex, different substrates are present in different cell types, and rapamycin modulates different tissues in slightly different ways.
Some researchers speculate that if rapamycin is given intermittently (e.g. every 5-7 days) the sustained inhibition of mTOR complex II can be circumvented – and with it the advent of metabolic adverse effects. However, this is just pure speculation and we do not know whether the positive vs. negative effects of rapamycin have anything to do with mTORC1 vs. mTORC2 inhibition and our model of how rapamycin brings about its effects (or side effects) is incomplete.
Other experience reports
For a full list of experience reports click here.
Sources & further information:
- Podcast: Peter Attia & David Sabatini – M.D., Ph.D.: rapamycin and the discovery of mTOR — the nexus of aging and longevity?
- Opinion article: Rapamycin for longevity: opinion article
- Scientific study: Rapamycin fed late in life extends lifespan in genetically heterogeneous mice
Disclaimer
The content available on this website is based on the author’s individual research, opinions, and personal experiences. It is intended solely for informational and entertainment purposes and does not constitute medical advice. The author does not endorse the use of supplements, pharmaceutical drugs, or hormones without the direct oversight of a qualified physician. People should never disregard professional medical advice or delay in seeking it because of something they have read on the internet.
