Leptin is a master control hormone that regulates long-term energy homeostasis. Leptin is not just a “satiety hormone” as many people wrongly assume, but leptin’s main role is to act as a “starvation signal” when leptin levels are low.
We are the only primates that have conspicuous fat deposits year-round, particularly females (e.g., buttocks, breasts). And when these fat deposits run low, our bodies evolved to shut down many physiological and mental processes. The signal for how “full” these fat deposits are is mediated by leptin. Therefore, leptin can be considered the body’s “fat report” as it is secreted by adipose cells in proportion to how “full” these cells are.
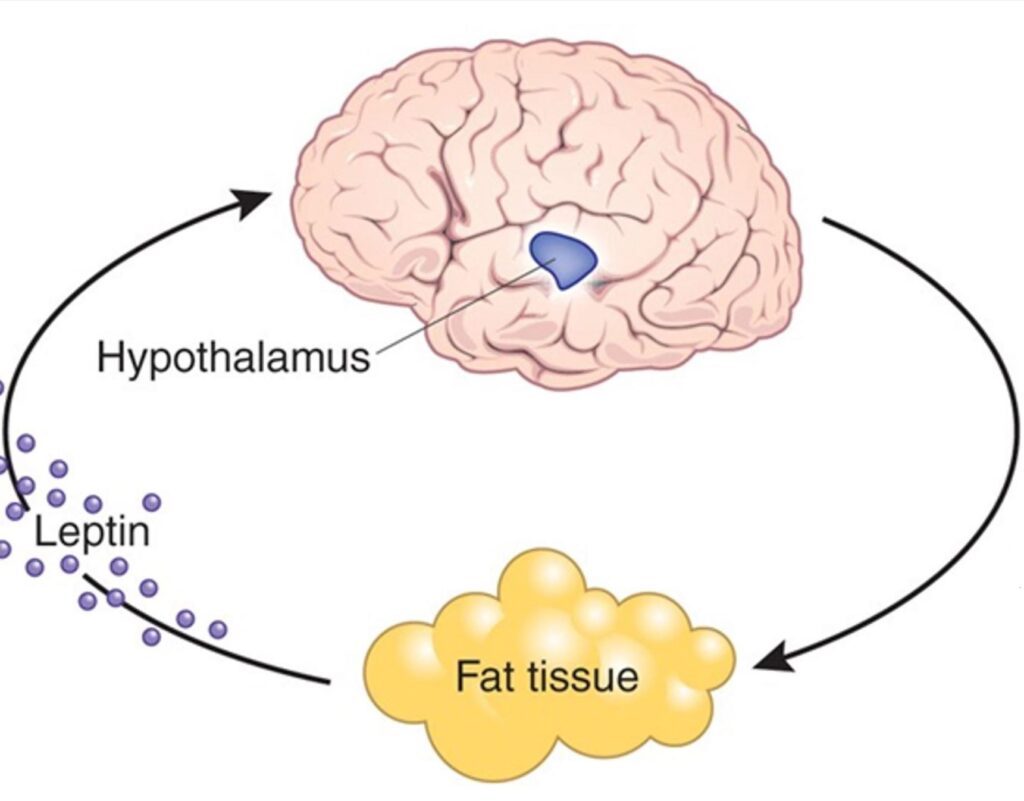
When leptin levels are adequate, this is a “go-ahead” signal for the animal to spend its energy in pursuit of other things besides acquiring food. If body fat levels fall below a certain threshold, leptin levels tank.
When levels get low, all aspects of the organism are driven toward the maintenance of sufficient fat stores for survival. This includes a shutting down of endocrine systems, reproduction, sympathetic nervous system activity, and non-food-related interests. At the same time, appetite, foraging behaviors, and a mental preoccupation with food are all ramped up.
It makes little difference whether leptin levels are “normal” or “high”, but it makes a huge difference if leptin levels fall below a specific threshold, progressively adapting the organism to starvation the lower leptin levels get.
Leptin receptors are expressed in a variety of tissues and organs, including the liver, immune system, and gastrointestinal system. Leptin receptors are even found on the tongue, more specifically taste buds, so that for a starved animal sweet foods taste even sweeter (regardless of leptin also influencing the hedonic response to foodstuff at multiple levels in the brain), which I find fascinating.
However, the major action is on the central nervous system. Leptin receptors are expressed all over the brain and when leptin levels are low, emotions, thoughts, behaviors, and the endocrine system are put into “starvation mode”.

Leptin is flying under the radar and I have never encountered a doctor who looks at leptin levels – not even endocrinologists or other hormone specialists. In my opinion, some unsolved problems with energy levels and hormone problems may be due to undiagnosed hypoleptinemia. Many try testosterone and thyroid replacement but still suffer from some symptoms of hypogonadism or hypothyroidism. Even if said hormones are normalized artificially, many of the symptoms remain if leptin levels are low.
Personal experience
Energy levels have a major say in my charisma, wit, how much fun I am to be around, mindfulness, cognition, productivity, and life enjoyment. But without enough leptin, my energy levels are awful – regardless of the levels of other hormones.
A few years ago I dieted down to very low levels of body fat, which I stupidly maintained for quite a long time. I was always lethargic, and it felt like somebody had pulled out my batteries. I also had problems with thermoregulation, my mood and libido were low, and simply moving around felt like an onerous task.
Furthermore, despite the lethargy, I was also quite restless, driven, and my mind was racing all of the time. I was plagued by an inability to sit still and had an irresistible urge to always be productive (presumably a proxy for “foraging behaviors”). I needed to exercise vigorously only to feel normal. I also became socially anhedonic and wanted to be left alone. A lot of my mental brainpower was taken up by food.
I started to replace other hormones, though this only partially helped. This was long before I knew much about leptin.
Recovery took a lot of time, a GLP-1 agonist, and some time on metreleptin (the only currently available leptin analog) to get fully back to normal, presumably because many parts of my brain trophically and epigenetically adapted to “starvation mode” and it took some time, and exposure to high leptin levels, for them to reverse back to “normal mode”.
At the time, my leptin levels were below 1 ng/ml (hypoleptinemia). While this may sound cliché, a couple of hours after my first injection it seemed like somebody had turned the lights back on. I felt a surge in energy levels, my mood became much better, my hunger levels declined, and I was in a distinct state of “presence”.

Before starting metreleptin, I experienced hunger every few hours, even while taking semaglutide (as semaglutide cant exert its effects well if leptin levels are low – as explained in more detail in my article on semaglutide). However, from the first day of using metreleptin, my post-meal fullness improved significantly – I was able to eat and then stay satisfied for many hours.
Furthermore, my dreams became ultra vivid (particularly during the first week), my sleep duration increased, and I felt deeply rested in the morning after waking up, something I had not done for a long time.
I was surprised by the rapidity of the effects, which were in fact immediate.
Interestingly, over the next couple of months, I needed to reduce the dosage by over 50% as the effect of metreleptin seemed to get stronger over time (i.e., I lost nearly all of my appetite and nothing seemed appealing), presumably reflecting trophic changes in the central nervous system. Over the next couple of months, I titrated my leptin levels in a way that would match the leptin levels of a male with 15-20% body fat.
On the physical side, the only thing I noticed was some fat redistribution. Despite not having lost body fat (I actively tried not to by elevating my caloric intake to well above 3000kcal/day), fat redistributed from the facial & trunk regions to my extremities, something that is both metabolically and aesthetically pleasing.
Adipose tissue specifically in my face decreased to the point where many of my friends & family commented on it, particularly my jawline became more pronounced, though my cheeks started to look a little gaunt, which caused me to lower my leptin dosage a second time. Metreleptin-induced loss of facial fat is also reported in the medical literature. Overall, my face became significantly more attractive (as judged by female friends and short-term dating success).
Replacing leptin essentially lowered my “fat setpoint”, allowing for maintaining very low body fat levels without suffering from lethargy, ravenous hunger, hormone deficiencies, and other starvation-related adaptations, which would obviously be of immense value to the modeling, acting, fitness, and bodybuilding industry.
Even after stopping the drug (I had been on it for roughly 2 years at a low dosage), I somehow “trained” my nervous system to stay at lower body fat levels. At the time of this writing (01/2025), it has been 2 months since I stopped taking the drug and the effect still holds. I will update in the future on whether this effect is sustained. This effect was interesting.
This is perhaps similar but in reverse to how many people need to overshoot their before-diet starting weight to fully “recover” from diet-induced adaptation. We know that the weight settling point can be shifted upwards (as is often the case with obesity) but thus far we have not able to permanently shift it downwards (even GLP-1 agonists only lower it while on the drug). I do think that the cytokine leptin is part of the puzzle.
While procuring the drug was a hassle, playing around with metreleptin was one of the most interesting and fruitful experiments I had ever done. I may write a more detailed article about my experience with metreleptin at some point in the future as this is something that, to my knowledge, nobody has done (or at least written about) before. To get notified when it is available, sign up for my newsletter.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Overall, it took many months of normalized eating, weight gain, and leptin exposure to feel “normal” again – probably accompanied by measurable brain changes in a number of regions. There is fMRI evidence that after 1 year on metreleptin, there are a number of trophic changes in brain structure.
Symptoms
- hormone imbalances
- low energy levels
- low sex drive
- ravenous appetite
- reactive hypoglycemia
- restlessness
- low sympathetic nervous system activity (e.g., low blood pressure, low heart rate, low body temperature, fatigue)
- persistent symptoms of multiple hormone deficiencies that do not adequately improve with hormone replacement therapy
Diagnostics
- Blood test: leptin
Interventions that worked for me and friends
- Metreleptin: The only currently available leptin analog. Mibavademab, a leptin receptor agonizing antibody, is currently in clinical trials though it is uncertain whether brain sites beyond the circumventricular organs are reached.
- Gaining body fat: Body fat levels are the major determinant of leptin levels. At low body fat levels, leptin levels are low. However, high body fat levels are often accompanied by leptin resistance. Most males feel and function best between 13-18% body fat.
- Semaglutide: Semalgutide is a known “leptin sensitizer”, which means that leptin signaling is stronger. Among other things, GLP-1 agonists are thought to increase the expression of leptin receptors on the order of 40-70% but presumably also enhance intracellular leptin signal transduction. Of note, for me, metreleptin allows me to keep my body fat low without starvation-related adaptations, something that semaglutide does not.
- Increasing caloric intake: It seems that caloric intake has an effect on leptin levels independent of body fat levels. Furthermore, it seems that a high caloric intake has a leptin-sensitizing effect in the hypothalamus, regardless of body fat levels.
- Increasing carbohydrate consumption: Insulin potentiates leptin synthesis.
- Avoid: ketogenic diets, intermittent fasting, multi-day fasting, dieting
Leptin vs. ghrelin?
There is a widespread misconception that leptin is the antagonist of ghrelin. This misconception implies that leptin and ghrelin play in the same league of importance. Nothing could be further from the truth.
Leptin is one of the few master control hormones, whereas ghrelin serves a few highly specific and to some degree expendable minor functions, exerting its effects on measly G-protein-coupled receptors. Leptin, on the other hand, exerts its effects via a special cytokine receptor, directly linked to widespread and major alterations in gene expression through STAT3 activation.

“I can maintain sub 10% body fat with no issues”
The human body evolved a mechanism that it stops working properly if adipose tissue reserves are too low, presumably for survival-related reasons. However, purely from a functional perspective, there is little reason to assume that humans need to carry more fat than the closely related chimpanzees.

Even though carrying a decent amount of adipose tissue is not needed from a functional perspective, the human body nonetheless evolved to “shut down” when adipose tissue is lacking by way of gradients in leptin signaling.
Every one of us has a different “leptin threshold” level and also a different level of leptin synthesis for a given amount of body fat. Furthermore, individuals differ in terms of leptin sensitivity. Consequently, some people can naturally stay at very low levels of body fat without adverse effects, whereas for others body fat needs to be much higher.
There are a couple of people who claim that they can maintain a sub 10% body fat “just fine”. I suspect that the vast majority of these people are either purposefully lying, have little self-awareness, or are so much used to their state that they have simply forgotten what being in “normal non-starvation mode” feels like.
Obviously, there are genetic differences in leptin secretion per fat mass and/or leptin receptor signaling and some people can truly stay at very low body-fat levels without suffering from starvation-related adaptations – but the vast majority of people cannot.
To test this, if somebody can truly maintain a sub-10 % body fat level “just fine” (or sub-18 % for females), he would not feel anything from metreleptin administration at a dose sufficient to mimic 20% body fat.
Unfortunately, many people do not really recover after dieting down to a body fat level too low (e.g. cold intolerance, anhedonia, loss of libido, lethargy) — online there are many anecdotes of people that seem to have permanently screwed themselves by dieting down too low. Even more unfortunate, leptin agonists (which are next to impossible to get hold of) are perhaps the only thing that can truly reverse these changes without a significant amount of fat gain.
The dangers of keeping body fat % too low
I recently dated a woman who has had amenorrhea for over 2 years. She also had cold hands and feet and a blood pressure of 105mmHg. Her SHBG was high. She felt tired most of the time.
Endocrinologists did not know what was wrong with her because releasing-hormone tests had shown that her hypothalamus and pituitary function perfectly well if they are stimulated.
She told me that a couple of years ago she had lost about 15kg of fat. Ever since, she maintained this weight through willpower. Why no endocrinologist (one of them a university professor) has picked up on that important detail is beyond me. The role of leptin in hypothalamic amenorrhoea has been well-known for a long time (study).
Similarly, about a decade ago, I dieted down to single-digit body fat levels (4.8% at my lowest point) and stayed around there for about a year (perhaps the biggest mistake I have made in my life). I did this mostly because of vanity reasons. Back then, my energy levels were abysmal and multiple hormonal axes were in the gutter. My mental health was much worse as well.
Weight regain and starting to eat 3000kcal/d have not fully reversed all of the starvation-related adaptations even years after the fact. Supraphysiological doses of “satiety” peptides (insulin; GLP1-agonists; metreleptin) mostly normalized things though only while I was taking them. As soon as I came off, many of the adaptations came back.
Of all the interventions I had tried, leptin administration was by far the most powerful.
After weight recovery, former patients with anorexia nervosa have all sorts of issues for a long time – even if they recover more than their original fat mass. For example, in one study, baseline noradrenaline levels as well as stimulated noradrenaline levels remained much lower even 4 years after weight recovery.
Similarly, after the biggest loser study, participants had a negative delta in their basal metabolic rate of about 500-600kcal per day (!) despite being back to their original fat mass. The only hormone that was much lower 7 years after the fact was leptin… (study).
It seems, that the brain somehow “remembers” periods of starvation (whether self-induced or otherwise) and that, for many people, these adaptations are quite stubborn and hardly go away in a similar way obesity hardly goes away after a person has become obese. (Of note, GLP-1 agonists only lower body weight/fat setpoint while the treatment is ongoing but as soon as people come off, most of the weight is regained).
How does this “imprinting” happen? There are two possibilities (at least, I cannot think of any other). Firstly, adaptations happen at the level of neural networks (long-term depression or long-term potentiation of existing networks). Secondly, adaptations happen at the cellular level (perhaps sustained alterations in the expression of certain transcription factors in key neuronal populations such as POMC/CART neurons presumably due to gene methylation).
In sum, maintaining body fat much below the fat set point for a long time seems to carry long-term risks. Some regain their weight and recover fully and quickly. Others are not so lucky. I have met quite a few people who did not recover properly – some did not even recover after becoming much fatter than they were.
I am currently writing an in-depth article on the topic.
Why I have not built antibodies to metreleptin
I discuss this in more detail here: Why I have not built antibodies to metreleptin
Sources & further reading
- Scientific study: Short-term metreleptin treatment of patients with anorexia nervosa: rapid on-set of beneficial cognitive, emotional, and behavioral effects
- Scientific review: The role of hypoleptinemia in the psychological and behavioral adaptation to starvation
- YouTube: Tristyn Lee – My Experience Maintaining 4% Body Fat for 2 Years
