Is it about “calories in” vs. “calories out”?
After starting a low dose of semaglutide, I needed to increase my caloric intake from about 2.400 kcal/d to about 3.000 kcal/d in order to not lose weight (which I did not want to – I only wanted to blunt my appetite). I needed to up my calories by another 500-800 kcal/d or so after adding a low dose of metreleptin. So, in total, I was eating about 1.000-1.300kcal more than before to maintain the same weight.
Interestingly, over the first couple of months on low doses of both of these drugs (0.2mg semaglutide/week + 1mg metreleptin per day), my body fat percentage dropped from 11.6% to 10.6%, while muscle mass was unchanged (as measured via DEXA scan). During this time, I did not (knowingly) change my exercise levels, though I did not measure fidgeting or other unconscious movements resulting in non-exercise-induced thermogenesis (NEAT).
Similarly, a friend of mine recently started on semaglutide for weight loss. He lost about 5kg “without even trying” in about 2 months. Given that his strength in the gym did not deteriorate, he speculates that most of the weight loss was due to fat loss (though he did not measure it). And given that one kg of human fat is worth about 7.000 kcal, he was losing weight much faster than what thermodynamics would have predicted.
Another friend claims that he was burning fat like crazy after adopting a ketogenic diet. While basal metabolic rate (BMR) is known to rise on keto, and in addition, a small number of calories are lost in the form of ketone bodies via the urine and breath, this is presumably not the whole story.
Another friend of mine seems to gain weight rapidly whenever she just slightly goes off her meticulously planned out diet. Water retention disregarding, it looks like years of yo-yo dieting have “programmed” her system to store everything in sight.
Similarly, if people are put on certain neuropharmaceutical drugs (e.g., antipsychotics, mirtazapine, phenelzine), many seem to gain weight much more rapidly than what “calories in vs. calories out” would predict. Gains of 5kg during the first month are not unheard of.
A lot of people assume that weight gain or loss comes down to energy in vs. energy out. According to that theory, people would need to consume about 7.000 kcal less or burn 7.000 kcal more to lose 1 kg of fat mass. However, in reality, the body is not a calorimetric machine, and things are not that simple. In all of these anecdotes above, the change in hormones or neurotransmitters seems to have altered either fat flux or brain energy homeostasis, or both.

After I put things into my mouth, I give up control of what happens to the foodstuff I have just ingested, and my body takes over.
While the laws of physics cannot be ignored and the ingested energy must go somewhere, surplus energy does not necessarily have to be stored. While calories in vs. calories out is still true in the most technical sense (you can’t argue with physics), what happens to the ingested energy depends to a large degree on the nervous system and the endocrine system. I will now discuss both separately.
(Just to clarify, calories do matter. However, calories are just part of the story.)
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
The nervous system and its role in metabolism, nutrient partitioning, and body weight
Semaglutide does not “only” help people feel full faster, and therefore eat less, as it is often reported – even by doctors. More importantly, it “reprograms” certain parts of the hypothalamus to assume a lower weight setpoint which then sets a cascade of counterregulatory mechanisms in motion. For example, anecdotally, people who had been watching their calories and exercise closely, now seem to be losing weight rapidly even if they do not change their caloric intake or exercise habits.
For a long time, the role of the nervous system in metabolism, nutrient partitioning, and body weight was neglected. Some zealots were claiming that it is all about “calories in vs. calories out”, while others were claiming that it is “all about insulin”. However, the introduction of GLP-1 agonists has impressively proven that both camps were very narrow-minded and that the nervous system is an equally important player – perhaps the most important player of all.
For example, if a certain part of the hypothalamus is lesioned, humans or animals start to balloon, and the ballooning is not solely caused by “pigging out”.
In the graphic below, a low dose of metreleptin was given to congenitally leptin-deficient individuals, leading to a weight loss of about 40% of body mass within 18 months, about twice as much as “normal” obese GLP-1 treated individuals (who lose about 20% of body weight within the same period of time).
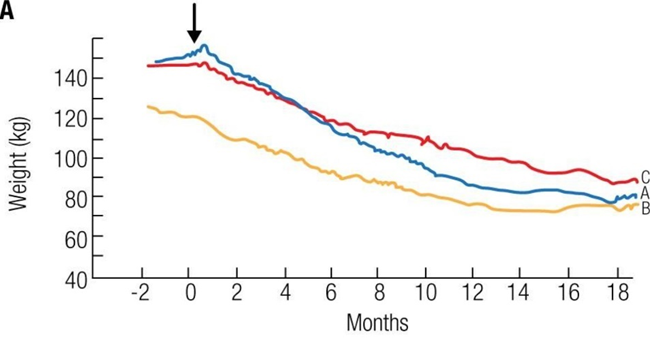
Even more impressive, a congenitally leptin-deficient boy was treated with mibavademab (a monoclonal antibody allosterically activating the leptin receptor).

It is hard to imagine that a 7-year-old boy with a BMR of probably around 1000kcal per day can lose 7.1kg of fat on average per month for 6 months purely through “eating less”.
Even though genetic leptin mutants are not representative of the wider population, the major point is that certain changes in the nervous system can dramatically affect the “weight settling point” in both directions.
Exactly how the nervous system mediates these effects on body weight is not entirely known. It is likely quite multifactorial, including modulation of sympathetic nervous system activity (which is a major part of basal metabolic rate), controlling the rate of lipolysis through noradrenergic innervation of adipose tissue, and the vagal control of hepatic metabolism and insulin release.
While many areas of the nervous system are important, it seems that a certain group of hypothalamic neurons, so-called POM-C neurons, is essential.
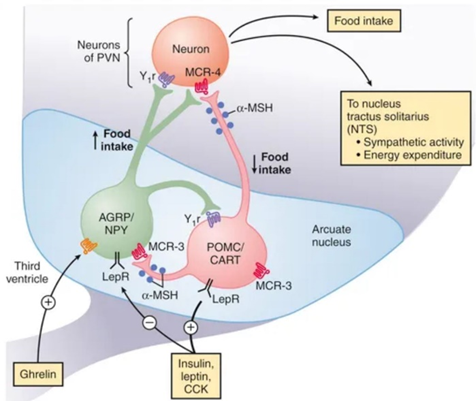
These neurons co-control the endocrine system, the autonomic nervous system, and the rest of the body through these two systems. For example, when POM-C neurons are downregulated (such as during a diet, intermittent fasting, or after multi-day fasting), hormones and activity levels decline, and people start to feel cold, hungry, weak, and preoccupied with food. They also rapidly gain weight because their body is primed to do so. This is the principle behind yoyo-dieting.
The sustained downregulation in POMC-neurons, and upregulation in AgRP-neurons, is also part of the reason people have a challenging time keeping off weight after they have lost it. The fact that GLP-1 agonists like semaglutide activate this group of neurons is probably the primary reason why GLP-1 agonists are such amazing weight-loss drugs (and in my opinion, perhaps even healthier and more sustainable than losing weight via the natural route).
The nervous system and the endocrine system (discussed next) exist in a state of intense crosstalk, and both influence each other. Nonetheless, it seems that the nervous system itself has a major say in what happens to the calories I ingest, and especially how much fat I carry.
I discuss my experience with semaglutide here.
The endocrine system and its role in metabolism, nutrient partitioning, and body weight
Metabolism in animals is primarily regulated by hormones. There are about thirty or so hormones that regulate metabolism in one way or another. I will center my discussion on insulin, which can be regarded as the master control hormone of metabolism.
Insulin is the “hormone of abundance”, and its job is to store energy. This goes far beyond the cellular uptake of glucose. Insulin stimulates the activity of hundreds of different proteins, and its downstream effects alter the expression of around 2000 genes.
In short, insulin stimulates whole-body anabolism (“growth”), while its absence induces whole-body catabolism, the latter of which is readily seen in Type I diabetics.
Insulin is a central regulator of a variety of metabolic processes. For example, it inhibits lipolysis (the breakdown of triglycerides in fat tissue) and beta-oxidation (the breakdown of fatty acids for energy), while stimulating lipogenesis (the synthesis of fatty acids) and fat storage (uptake of fatty acids into adipocytes and their reesterification into triglycerides).
Let’s say there are two identical twins eating 2500 calories with the exact same macronutrient composition. If we give one of the twins a decent dose of insulin, the individual receiving insulin will gain a lot more weight than the other person, even if their diets (& genetics) are otherwise equal.
Whenever insulin levels are high, the body is in “storage mode”, and ingested fats are readily stored. Conversely, whenever insulin levels are low (such as on a ketogenic diet), triglycerides are readily liberated from adipocytes, and the hydrolyzed fatty acids are turned into ketones or burned for energy.
A couple of years ago, I remember reading an article about “diabulimia”. “Diabulimics” are people, usually females, with type I diabetes who stop or drastically reduce insulin injections to lose weight. In many cases, weight loss is quite drastic and seemingly much faster than what “calories in vs. calories out” would dictate (even if one accounts for glucosuria – glucose lost via the urine).
Conversely, in another study, the insulin dose was rapidly increased from zero to about 100 IU per day over the course of six months (for reference, a normal daily physiological production of insulin is about 40-60 IU/d). During this time, the participants gained about twenty pounds of fat, but the ingested calories dropped by about 200-300 kcal per day. The only difference was profound hyperinsulinemia.
Therefore, how the ingested energy is handled depends in part on how the ingested food affects insulin levels. I discuss insulin and insulin resistance in more detail here: Insulin, Insulin Resistance, and Strategies I Use to Keep Insulin-Sensitivity High
Other hormones that influence metabolism, nutrient partitioning, and body weight
The major hypothalamic hormones (testosterone, estradiol, thyroid hormones, cortisol, growth hormone) all heavily influence basal metabolic rate (BMR) through a variety of mechanisms. However, these hormones also co-determine what happens to the calories I ingest (nutrient partitioning). Hormones & metabolism are a huge topic, so I limit my discussion to the major hypothalamic hormones.

- Thyroid hormones: T4 and T3 regulate “metabolic speed”. Thyroid hormones increase the general rate at which metabolic processes are happening and therefore their effects can either be anabolic or catabolic, depending on caloric intake and the presence of anabolic hormones. Generally speaking, hypothyroid individuals have a hard time losing weight, whereas hyperthyroid individuals lose weight easily.
- Stress hormones: Cortisol prepares the body for stress. It stimulates gluconeogenesis & lipolysis and turns off anabolic pathways. In the short term, cortisol is catabolic to everything (muscle, fat, connective tissue, nervous tissue), whereas, in the long term, cortisol is anabolic to fat tissue in the face & trunk while remaining catabolic to everything else.
- Sex hormones: Testosterone and estradiol also regulate nutrient partitioning. Testosterone is anabolic to muscle and catabolic to subcutaneous fat, while estradiol stimulates the deposition of subcutaneous fat (presumably to prepare the organism for pregnancy). Both hormones also increase insulin sensitivity.
- Leptin: Leptin adapts the organism to starvation. In its absence, the synthesis of other hypothalamic hormones (except for cortisol) is vastly reduced. Furthermore, what is not known to many, leptin directly regulates the metabolism of the liver, muscle, and fat (all of which together make up about 80% or so of body mass).
- Growth hormone is catabolic to adipose tissue and anabolic to everything else.
- IGF-1 is anabolic to everything.
I discuss my personal experience with these hormones in more detail here.
Why do I mention these hormones here given that they can hardly be changed (short of exogenous hormone replacement)?
Insulin levels affect all of them (and insulin in turn is affected by what I eat). For example, insulin increases hepatic and renal conversion of T4 to T3, induces hepatic IGF-1 synthesis, increases leptin expression, and promotes hormone bioavailability by regulating levels of hormone-binding proteins (e.g., SHBG, CBG, TBG, IGFBP-3).
In sum, different diets affect different hormones differently. These hormones then have different effects on energy metabolism, fuel partitioning, body composition, weight gain and loss, and the nervous system.
Hacking the system with metabolic drugs
There are a variety of metabolic hacks that can affect either the nervous system or hormones or both. These include acarbose, SGLT-2 inhibitors, metformin, and GLP-1 agonists such as semaglutide. I discuss my experience with metabolic drugs in more detail here.
Related articles
Sources & further reading
- Podcast: Peter Attia AMA #22: Losing fat and gaining fat: the lessons of fat flux
- Scientific study: Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo
Disclaimer
The content available on this website is based on the author’s individual research, opinions, and personal experiences. It is intended solely for informational and entertainment purposes and does not constitute medical advice. The author does not endorse the use of supplements, pharmaceutical drugs, or hormones without the direct oversight of a qualified physician. People should never disregard professional medical advice or delay in seeking it because of something they have read on the internet.
