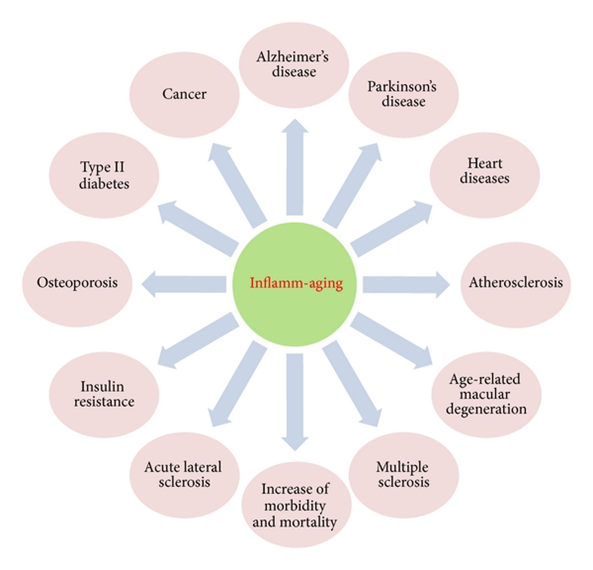
Over the last couple of years, more and more research has come out on how inflammation affects (and drives) a variety of conditions, ranging from dementia, osteoporosis, atherosclerosis, and depression.
Chronic inflammation has emerged as a key feature of aging and age-associated diseases (“inflammaging”).
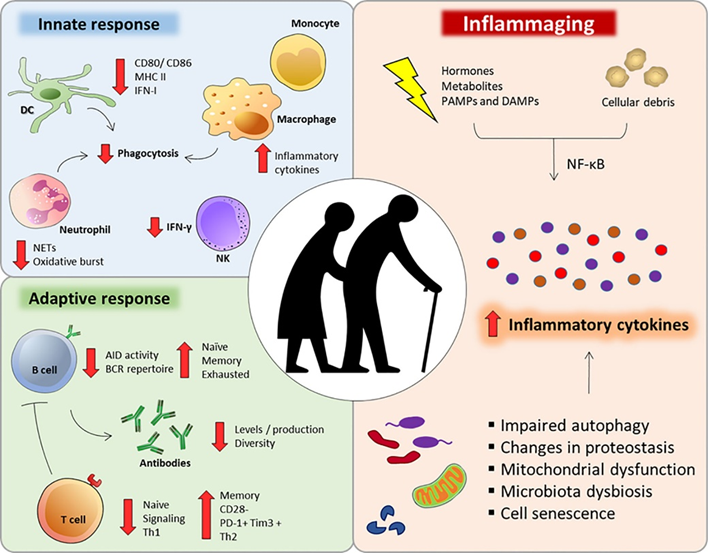
Age-related inflammation is thought to be caused by a variety of things:
- Senescent cells and their senescence-associated phenotype (SASP)
- Progressive accumulation of intracellular and extracellular waste products (e.g., advanced glycation end products, metals, particulate matter, microplastics, lipofuscin, etc.)
- Mutations to mitochondrial DNA (mitDNA) and mitochondrial breakdown
- Senescence of the adaptive immune system (immunosenescence) due to the depletion of hematopoietic stem cells and senescence of already existing lymphocytes, which causes the adaptive immune system to be hypofunctional, resulting in a hyperfunction of the innate immune system
- The herpesviruses EBV and CMV constantly trying to break out, which is followed by immune system activation. Beyond 60 years of age, roughly half of the human immune system is constantly busy with fighting CMV.
Because of these and other factors, background levels of inflammation keep rising as age goes up. Inflammation is then thought to harm tissues in at least three ways:
- Cytokines (inflammatory mediators) affect the function of healthy cells in a negative way. Particularly IL-11 has recently come out as a key player and preliminary data suggests that IL-11 blockade results in a lifespan extension of roughly 25%.
- Cytokines and inflammation-associated growth factors stimulate fibroblasts to build connective tissue. This build-up of extra connective tissue (i.e., fibrosis) affects the structural integrity of tissues.
- Cytokines hamper metabolic health directly by changing the activity and metabolism of several tissues.
Tactics I follow to keep inflammation levels down
Tactics I follow to keep inflammation levels down
- Diet
- Daily exercise
- Optimizing metabolic health
- Keeping body fat low
- Rapamycin
- Counteracting the accumulation of senescent cells
- Antioxidants
- Niacin
- Beta-alanine
- Counteracting the accumulation of heavy metals
Diet
I follow a diet that is quite clean and rather low in pro-oxidative ingredients. During the day, I drink a lot of Huel shakes with olive oil. I discuss my diet in more detail here.
Daily exercise
I exercise daily. I discuss my exercise routine in more detail here: My Fitness Protocol
In the short term, exercise leads to a burst in oxidative stress due to the vast increase in metabolic rate. However, in the long term, exercise lowers inflammation (as measured in the form of lipid peroxidation and certain cytokines), presumably due to a counterregulatory upregulation of antioxidant systems and oxidative damage repair systems. It is thought that this effect is specifically pronounced with high-intensity exercise.
Hence, through defense mechanisms aimed at countering exercise-induced oxidative stress, cells and tissues in individuals who regularly exercise demonstrate reduced baseline oxidation levels. This concept is known as hormesis.
I personally end each zone II cardio session with 7 minutes of high-intensity exercise, which though is probably not sufficient for reaping the full benefits.
Optimizing metabolic health
I optimize metabolic health (see here).
Among other things, I try to keep the amount of visceral adipose tissue (a consequence of insulin resistance, alcohol, sugar, and cortisol) quite low as it drives inflammation and insulin resistance through the elevation of local free fatty acids and the secretion of proinflammatory adipokines and cytokines. I use a low dose of metreleptin to help with this.
Furthermore, I try to keep glucose excursions low because glucose spikes lead to the generation of advanced glycation end products (AGEs), which are then recognized by macrophages as a pro-inflammatory signal. I do this by eating/drinking a lot of Huel shakes and trying to keep high-starch meals to once per day (though with a lot of room for flexibility). On and off, I use a continuous blood glucose monitor for this.
I also experiment with a host of metabolic drugs. I currently take allopurinol. On and off, I also take microdoses of semaglutide. I discuss my experience with metabolic drugs in more detail here.
Lowering the activity of the mTOR pathway with rapamycin
mTOR activity stimulates inflammation, and inflammation stimulates mTOR activity. Rapamycin is pretty good at reducing age-related sterile inflammation. I take 4-5mg per week. From November to January each year I take a break, partially also because during this time people tend to get sick the most.
I discuss my experience with rapamycin in more detail here.
(By now it should be clear that many of the tools & tactics I use are beneficial for a variety of different strategies. For example, rapamycin keeps my cells from senescing, improves metabolic health (if dosed weekly), sharpens the adaptive immune system, reduces inflammation, improves cellular and tissue health, and counteracts carcinogenesis.)
Counteracting the accumulation of senescent cells
Senescent cells are proinflammatory because of the proinflammatory mediators they secrete. Rapamycin is particularly powerful in slowing the accumulation of senescent cells. Furthermore, I tried to clear some of my tissues of senescent cells by recurrent administration of dasatinib & quercetin (D&Q). I discuss my experience with senolytic drugs in more detail here.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Antioxidants
Ever since oxygen accumulated in the Earth’s waters and atmosphere about 2.5 billion years ago, the vast majority of complex life on Earth evolved to use oxygen for its metabolism. Unfortunately, the same oxygen is highly reactive and can damage all kinds of molecules. Thus, organisms evolved a variety of antioxidant defense mechanisms. When this balance is disrupted, it is called oxidative stress.
Reactive oxygen species (ROS) then damage cellular components, including lipids, proteins, and DNA. This then leads to dysfunction and inflammation. Furthermore, some of these destroyed components can hang around for years and decades (e.g., crosslinked collagen, lipofuscin, protein aggregates), impairing proper tissue function.
ROS specifically lead to mitochondrial damage because most ROS are generated in the mitochondria. This is one reason why the mutation rate of mitochondrial DNA is somewhere between a hundred and a thousandfold faster than nuclear mutation rates.
Antioxidants are molecules that either prevent reactive oxygen species from being formed or remove them (usually by directly reacting with them), thus minimizing their damage.
I take a couple of antioxidants in low doses. These include N-acetylcysteine (300mg), alpha lipoic acid (100mg), vitamin C (250mg), coenzyme Q10 (60mg), and selenium (100mcg). I discuss the supplements I have been taking now for roughly 5 years in more detail here.

Because ROS leak serves some physiological functions, including participation in intracellular signaling pathways, I am quite unsure about the benefits vs. harm of antioxidants. While antioxidants are perfect on paper, animal trials with antioxidants give conflicting results. I feel slightly less uncomfortable taking them vs. not taking them.
Another antioxidant (potentially) worth mentioning is resveratrol. However, in every experiment to date, resveratrol at several doses has failed to extend the lifespan of lean, genetically normal mice (with the exception of mice that were dying from a specific form of lipid poisoning due to being fed a high-fat diet).
Niacin
In the past I used to take niacin once daily (250mg – flush version). Niacin acts on the HC2A receptor (the same receptor ketone bodies act on) and has a variety of anti-inflammatory effects in a variety of tissues. However, niacin can cause insulin resistance for the next couple of hours, which is why I have stopped taking it.
Beta-alanine
I take beta-alanine (1g/d), which converts into carnosine. Carnosine not only protects against exercise-induced lactic acid production (as it buffers cells against changes in pH), but it also has metal-chelating effects, and some anti-glycation effects.
Glycation generates advanced-glycation end-products (AGEs), which are pro-inflammatory because they are recognized by macrophages as waste products (via the aptly titled “RAGE” – Receptor for Advanced Glycation End-products).

Counteracting the accumulation of heavy metals
Cadmium, lead, and mercury have no essential biochemical roles but exert a number of toxicities in multiple organ systems, in part because they create oxidative stress, which drives inflammation, and in part because they are mistaken by enzymes for essential cations such as calcium, magnesium, zinc, copper, and manganese.
I (try to) prevent the accumulation of heavy metals by taking a couple of supplements with metal-chelating effects (alpha lipoic acid, n-acetyl cysteine, beta-alanine, taurine). Some people claim that chelation therapy with EDTA or DMPS is much more effective. However, clinical data are lacking. Furthermore, some researchers speculate that EDTA and DMPS therapy frees heavy metals from bones, which are then inadvertently redistributed to the brain.
Bonus section: Inhibition of IL-11 signaling extends mammalian healthspan and lifespan
Lately, monoclonal antibodies targeting certain proteins have been all the rage. For example, antibodies targeting TNF-alpha have transformed the treatment of a variety of autoimmune diseases for the last 20 years or so. Or dupilumab (Dupixent), an antibody targeting interleukin 13 & 14, has transformed the treatment of atopic dermatitis and asthma.
Recently, an exciting paper was published. The authors blocked interleukin 11. Thus far, interleukin 11 has been elusive. It is thought that IL-11 mostly works in a paracrine fashion, meaning that it mostly acts locally on neighboring cells. When there is inflammation, among thousands of other things, IL-11 is secreted by cells to increase growth and inflammation in nearby cells, thus serving as an “inflammation amplifier” of sorts.
This makes sense in the context of injuries or infections. However, this is detrimental in the context of aging. Due to mitochondrial damage, accumulation of waste products, increased number of senescent cells, CMV and EBV trying to break out in a state of immunosenescence, etc. inflammation increases with aging.
In fact, aging is called “inflammaging” for a reason. The basic idea is that when one blocks IL-11, one decreases inflammatory processes, which are often associated with growth pathways (e.g., mTOR activation, AMPK downregulation, etc.). Therefore, by dampening the inflammation and growth through IL-11 blockade, this should theoretically increase health span and lifespan.
Lo and behold, in the paper published 10 days ago, lifespan and health span increased in a similar way as they do when rapamycin is administered (by roughly 25%). This finding, if confirmed by other laboratories (such as the Intervention Testing Program) is huge. I am eagerly awaiting results in genetically heterogenous mice, primates, and ultimately the approval for a human anti-IL-11-antibody (e.g., for fibrotic diseases).
My Longevity Protocol (Long & Technical Version)
This article is part of a much larger post describing my complete longevity blueprint. For my full protocol, read here.
Sources & further information:
- Scientific article: Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty
- Scientific article: Low-Grade Systemic Inflammation Connects Aging, Metabolic Syndrome and Cardiovascular Disease
- Scientific article: Chelation: Harnessing and Enhancing Heavy Metal Detoxification—A Review
Disclaimer
The content on this website represents the opinion and personal experience of the author and does not constitute medical advice. The author does not endorse the use of supplements, pharmaceutical drugs, or hormones without a doctor’s supervision. The content presented is exclusively for informational and entertainment purposes. Never disregard professional medical advice or delay in seeking it because of something you have read on the internet.
