I am in my early thirties. I want to live a long and happy life. Unfortunately, like most other things in this universe, my body is subject to a slow but progressive deterioration. In this article, I lay out my strategies for combatting the degenerative process called aging.
Some of the strategies I employ include:
- I put a prime on sleep, diet, and exercise.
- I optimize my hormones.
- I downregulate the mTOR pathway.
- I take pharmaceutical drugs aimed at improving metabolic health.
- I employ several behavioral, hormonal, and pharmaceutical strategies to delay cancer, atherosclerosis, and neurodegeneration.
- I cleared my body of (some) senescent cells with senolytic drugs.
- I monitor dozens of health markers.
- I take a handful of supplements.
- I put a prime on my mental health.
In this article, I will discuss the science, rationale, risks, benefits, side effects, costs, and much more.
Disclaimer
The content available on this website is based on the author’s individual research, opinions, and personal experiences. It is intended solely for informational and entertainment purposes and does not constitute medical advice. The author does not endorse the use of supplements, pharmaceutical drugs, or hormones without the direct oversight of a qualified physician. People should never disregard professional medical advice or delay in seeking it because of something they have read on the internet.
Part I. A Brief History of Human Lifespan
As long as early human tribes were small, infectious diseases were limited, and population growth for my early ancestors was restrained by the same phenomenon that restrains growth among other animals – limited resources and predation. In other words, not having enough to eat or being eaten.
As humans banded into ever-larger tribes (50-150 people), susceptibility to predation by carnivores became much less of an issue because together we were strong.
Thanks to the unique ability of our species for collective learning, hunting techniques have become more and more sophisticated. It did not take long before my ancestors became apex predators, hunting down the large animals around them. Everywhere humans appeared, this coincided with a large decline in the local megafauna.
For example, around 50.000y ago, there was a large decline in a wide variety of large African animals. Consequently, some humans expanded into Eurasia, where modern humans appeared around 20.000y ago. The same thing happens again: megafauna die-off (e.g., mammoth, woolly rhino).
Some humans crossed over into the Americas, where, again, the large animals suddenly started to magically disappear quickly after our ancestor’s arrival (e.g., glyptodont, giant ground sloth).

Because of the widespread (and likely anthropogenic) megafauna extinction, early humans were forced to find alternative sources of energy. Therefore, many tribes settled – not because it was a smart thing to do but because they had to.
Over the next 5.000 years or so, humans turned to agriculture at least ten times independently. After some early rough times, agricultural technologies got better, and the agricultural revolution eliminated the second major cause of death in animals: limited resources.
Population growth exploded, population densities increased, and wild-living animals were domesticated (e.g., pigs, sheep, cattle, goats, horses, fowl, etc.). On top of that, sanitary conditions in our early cities were not exactly sanitary. There was a lot of crowding, human and animal excrement, waste, and proper hygiene had not yet been “invented”.
Even though the resource restraint began to loosen, high population density, domestication of animals, and literally shitty conditions were a breeding ground for another restraint, which will remain the major cause of human death for the next 10.000 years all the way until the middle of the 20th century: infectious diseases.
Viral infections such as measles, smallpox, and chicken pox, mostly came from the animals we had domesticated. They decimated human tribes many times before humans gradually evolved to be more resilient and the pathogens evolved to be less virulent.
Many of these viral diseases became endemic and transformed into “childhood diseases”, which were regarded as a rite of passage to adulthood. Mostly because of these, back then about half of all children died before the age of 10.
Next to viral infections, also bacterial infections became more frequent, including typhus, typhoid fever, plague, and whooping cough, among others. Unlike viral childhood diseases, they could kill humans at any point in life, and outbreaks were presumably common.
Throughout human history, it was probably not the norm that someone made it past the age of 50 without having been killed by an infectious pathogen first. Then, in the 19th century, germ theory emerged, and vaccinations began.
To illustrate what that meant in numbers, let’s consider the example of smallpox. While we do not have exact data, it is estimated that between 1900-1950 smallpox caused about 400 Mio. deaths – four times as many deaths as World War I and World War II combined (which also happened in the same time period). After a vaccine was found, within a decade or so, smallpox was pretty much eradicated.
Around the same time, antibiotics were developed. Thanks to these “silver bullets”, major killers such as the plague, tuberculosis, pneumonia, and sepsis were, comparatively speaking, pretty much eliminated over the course of a decade also.
My grandma once told me that she was ridiculed by other women in her village because she did not have a “little angel” (where I am from, “little angel” is a term used for a deceased child). Back then, it was common, and even expected, that at least one of one’s children would die, and then protect the rest of the family from heaven.
Improvements in hygiene and sanitation, the onset of vaccination, and the development of antibiotics slashed the mortality from infectious diseases from the high double-digit %-range to the low single-digit range, in the process more than doubling (average) lifespan within less than a single century (after about 2000 centuries of Homo sapiens’ history).

Furthermore, with the development of modern farming techniques, the Haber Bosch process, and GMOs, humans ramped up food production by an ungodly amount, being the final straw for limited resources as a restraint to the human population and lifespan.
In sum, humans eliminated all of the major usual causes of death of “normal animals” (limited resources, predation, infectious diseases) through collective learning. However, even though this more than doubled the average human lifespan, this has done quite little for our maximum biological lifespan. In fact, the latter remained mostly untouched.
Predation, infectious diseases, and limited resources usually killed humans long before they could have died due to natural aging and its manifestations. Therefore, there was never an evolutionary pressure to keep humans healthy and well-functioning into old age.
While the average lifespan is still increasing by about three months per year, most of these “additional” years are thanks to a medical system well capable of prolonging the suffering from the manifestations of the aging process.
When external causes of death are excluded, deaths from “natural” causes increase exponentially with age, resulting in a doubling of mortality risk around every 7-8 years, approaching a 400-fold increased risk of death per year for a 100-year-old compared to a 20-year-old.

Nowadays, a sizeable percentage of all deaths are due to aging itself. While humans do not die directly of aging, one could argue that 80% of people die of age-associated diseases (at least in industrialized nations):
- Cardiovascular disease (50%)
- Cancer (25%)
- Neurodegeneration (5-10%)
These three slayers are intricately linked to a single underlying pathology: the aging process, which is currently the major threat to human lifespan because it indirectly makes people sick. However, even without these, human lifespan is limited by aging itself. It’s been estimated that if we abolish cancer in people, human lifespan goes up by only 3%. If you abolish atherosclerosis and cancer it’s about a 7% bump. If you got rid of cancer, heart disease, stroke, and diabetes, it’s about an 18% bump.
Evolution builds what is necessary, and that is it. If there had been a strong evolutionary pressure for humans to live well beyond 100 years, our ancestors may have evolved to do so. How much energy is invested into fighting the aging process depends on the reproductive success of an individual into old age vs. the energy costs associated with this.
However, past humans often did not make it past middle age and most of the reproduction occurred before the age of 40 or so. Therefore, there was never a need to keep human bodies well-functioning beyond that age because by that time reproductive success was approaching zero anyway (and still is – at least for females).
Therefore, from a tradeoff perspective, it does not make much sense to invest much energy into costly repair processes because the energy could be spent elsewhere (e.g., growth processes, immune function, reproductive efforts).
If the goal is to increase lifespan beyond what it is now, age-associated diseases need to be delayed, ideally by both tackling them individually and by tackling aging itself. How I personally go about doing this will be the topic for the rest of the article.
Sources & further information
- Book: Guns, Germs and Steel: The Fate of Human Societies
- Scientific article: Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition
- Scientific article: History of Vaccination
Part II. What is aging and why do organisms age?
What is aging?
Aging is the degeneration of an organism over time.
How do animals age?
Some researchers in the field try hard to synthesize a “theory of everything”: finding a single process, the ultimate root cause for what causes aging. Some try to explain it by progressive epigenetic methylation, others blame mitochondrial disease, others stochastic mutations, others the gradual accumulation of senescent cells, others the sustained havoc from oxidative damage, and others the relentless activity of the mTOR-pathway.
Our brains are meaning machines. We humans try to look for a simple, single, causal, nice & neat little sequence of events that explains things. Unfortunately, this is not how the universe works. Often, in nature, there is no single cause.
Rather, more often than not, there are several independent things happening in parallel – influencing, cross-interacting, and driving each other. Parallelism is something our brains are awfully bad at grasping because they evolved to cognize linearly in space and time.

Likewise, aging is (most likely) not caused by a single process. Aging itself is an emergent property caused by a multitude of independent (but interrelated) processes. I age at the macroscopic level because my cells and tissues age at the microscopic level.
But what exactly causes our cells and tissues to age at the microscopic level? While science has not figured it out exactly, it is highly likely due to a combination of independent processes including:
- Progressive degradation of the information contained within DNA: Stochastics genetic mutations, and particularly epigenetic modifications such as DNA-methylation, cause the genetic blueprint (mutations) or its readout (epigenetic alterations) of cells and mitochondria to be faulty. These changes can be caused by several things including stochastic chance events, radiation, reactive oxygen species, problems with the DNA-repair and/or maintenance machinery, among others. This is known as the information theory of aging but it is presumably far from the whole story.
- Progressive accumulation of senescent cells: Cellular senescence can be compared to “cellular zombification”. When a cell transitions to become senescent, it becomes dysfunctional, stops dividing, but also does not die. As we age, senescent cells accumulate throughout the body. They fill up cellular niches and prevent new, healthy cells from taking their place. Furthermore, they take on an inflammatory phenotype (SASP), which itself adds fuel to all of the other aging processes. Cellular senescence can be induced by a number of things including telomere attrition, overactivation of the mTOR pathway, endoplasmic reticulum stress, or a high mutation load.
- Gradual loss of stem cells: In a normal, young, healthy organism, a large number of stem cell niches are distributed throughout the body. These stem cells help to maintain a healthy population of normal cells. However, as stem cells are progressively lost, vacant cellular niches cannot be repopulated, and dysfunctional tissues cannot be properly repaired. Loss of stem cells can be stochastic (a function of time) or due to everything that kills normal cells, such as macro or micro-injuries, infections, radiation, mutations, or hypoxia.
- Waste accumulation: As time goes on, more and more unclearable waste will progressively accumulate both intracellularly and extracellularly throughout the body, and there is little we can do about it. This waste takes up space, drives inflammatory reactions, and impairs tissue structure and function. These wastes include metal deposits, microplastics, aggregates of metabolic waste (e.g., plaques, lipofuscin, inclusion bodies), and microscopic foreign bodies.
Aging through these and other processes is natural, spontaneous, and progressive. Every single one of these processes would suffice to drive at least some form of degeneration of the organism over time (i.e., aging) by itself – even if by some magical technology we would be able to eliminate all others.
Downstream, these processes also cause other “hallmarks of aging” such as loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, and altered intercellular communication.
These independent (but interrelated) mechanisms all fuel each other via various feed-back and feed-forward loops, meaning that, to some extent, they all drive each other. While all of these are important (and some of these are possibly more important than others), the truth is, they probably all play a role.
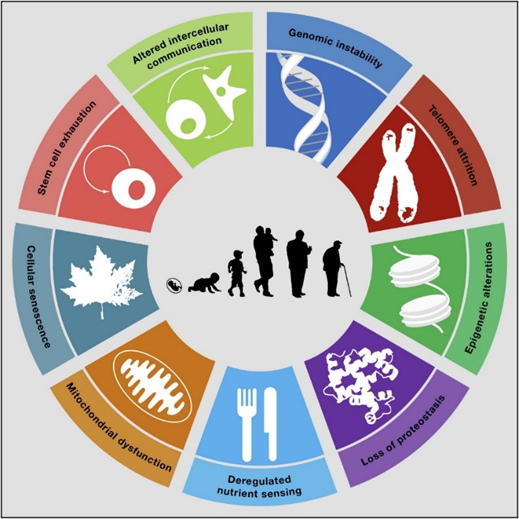
Since aging is caused by multiple processes taking place somewhat independently, it also answers the question of whether it is possible to cure aging. Unless we were able to somehow eliminate all of the processes involved 100% of the time (which is 100% utopian), at least some degeneration of the human body would continue to occur.
This means that no matter what I do, and no matter whether I like it or not, one or more of these processes will relentlessly progress, and I will ultimately die.
Animals (including humans) are multicellular organisms
All of these above-mentioned processes and mechanisms are happening at the level of single cells. However, as animals, humans are multicellular organisms. Our highly elaborate, wonderful vertebrate physiology relies on many different highly specialized cells, tissues, and organs to carry out specific processes that cannot be carried out by any other cell, tissue, or organ.
What good is it to be perfect at the cellular level, if the cardiovascular system, immune system, nervous system, liver, kidney, lungs, or gastrointestinal system are damaged for whatever reason (e.g., cancer, injury, infection, toxicity, trauma)?
Every one of these organs is of existential importance to the proper working and survival of the human body. Therefore, even if we were able to 100% eliminate all of these above-mentioned cellular drivers of aging, the degeneration or damage of a single, vital organ system would have massive deleterious downstream effects on the rest of the organism. Whether this single-organ degeneration is a consequence of the cellular drivers of aging, infarction, infection, cancer, or injury, does not matter.
For example, if my kidneys get damaged (e.g., by drug-induced nephrotoxicity, hypertension, hyperglycemia, autoimmune pathology), this will lead to damage, malfunction, and degeneration, either leading to death directly (e.g., by uremia), or at the very least by accelerating one or more of the cellular drivers (e.g., by rapid accumulation of toxic waste products). Therefore, I would age and die, unless kidney function is restored. Analogous things hold true for the other organs.
How to fight aging
From the last section, it should be evident that, when it comes to fighting aging, we need to employ two separate strategies:
- We need to tackle cellular aging from multiple, independent angles.
- We need to take care of the health of our individual organs. This means trying our best to protect them not only from cellular aging but also to protect them from injuries, toxicity, infection, or cancer.
Why do humans age?
Aging is spontaneous, progressive, and happens to everyone. However, is it mostly just an entropic process (i.e., a byproduct of being alive) or is it a feature specifically programmed by evolution? Anti-aging researchers fall into one of these two camps (natural aging vs. programmed aging).
Natural aging
Natural aging is the classic concept of aging: Many processes driving the aging process are simply a byproduct of being alive that results in progressive “wear & tear”.

More technically, this includes the perpetual pile-up of waste products throughout cells and tissues, sustained accumulation of mutations and epigenetic alterations, the progressive loss of stem cells, and the ever-increasing number of senescent cells.
For example, the foods I eat every day will deposit metals (and other non-degradable particles) throughout my body, over time progressively impairing tissue health and function. The air I am exposed to will result in the uptake of particulate matter such as microplastics or clumps of carbon. The sun’s rays hitting me every day will cause oxidation and crosslinking of macromolecules such as collagen or DNA. Etc., etc.
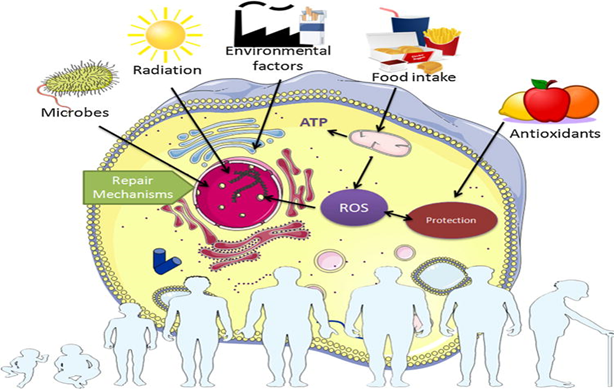
This process of natural aging is a slow and unavoidable consequence of existing in a universe driven by the maximization of entropy. However, even if I were living a perfectly sheltered life in sterile laboratory conditions devoid of pathogens, natural ground radioactivity, solar rays, particulate matter, environmental toxins, particles, trace amounts of heavy metals, etc., not even then could I avoid the wear & tear.
For example, because the DNA-replication & repair machinery are not bulletproof, random errors during cellular housekeeping will result in an increased mutational load over time. Combined with the stochastic DNA-methylation processes, this will result in an increasingly compromised readout of my genetic blueprint.
Furthermore, with each cell division, my limited telomeres will erode, eventually stopping the cell from dividing altogether. Therefore, even if no wear-and-tear from external conditions would occur, simply the failure of perfect housekeeping makes my organism essentially a ticking time bomb.

Programmed aging
In addition to the spontaneous, unavoidable “natural aging”, there might be a programmed factor on top (not mutually exclusive with natural aging). Programmed (or adaptive) aging refers to the idea that humans and most complex organisms may be equipped with biological mechanisms that purposely limit their internally determined lifespans beyond a certain species-specific age and that these mechanisms are evolutionary adaptations because aging creates an evolutionary advantage.
Said another way, the concept of “programmed aging” means that there may be specific mechanisms in place that actively drive the aging process on purpose to cause damage, degeneration, and death, in a similar way engineers do for products in the form of “planned obsolescence”.
But why would evolution come up with something like this? I explain this in more detail in my article on Programmed Aging. Even though I personally find it incredibly interesting, understanding this is not needed to understand the rest of the article.
What determines a species’ lifespan?
Aging happens spontaneously and to fight it, energy has to be invested. In most species, aging is not heavily fought by evolution due to the simple fact that individuals of most species die naturally quite early anyway. Thus, it doesn’t make too much sense to invest a lot of energy into the costly repair machinery. How much energy is invested depends on the gain in reproductive success if individuals of a species are long-lived vs. the energetic costs associated with it.
Therefore, how long a species’ lifespan is, is all about net reproductive success and how much a long lifespan would contribute to conceiving (and raising) viable offspring.
- If most individuals of a species die naturally early in life (e.g., predation, starvation, infection), there are few evolutionary pressures to evolve better mechanisms to fight entropy (e.g., better DNA-repair machinery, stem cell maintenance, waste removal, etc.). Consequently, in these species, the maximum biological lifespan is generally short simply because evolution never bothered to extend it.
- If members of a certain species are long-lived and reproductive success remains high for older individuals (e.g., bats, elephants, dolphins, humans, great apes, etc.), then it made sense for evolution to build a better, but costly, repair-machinery because increased reproductive success in old age outweighs the energetic costs.
- Very rarely, there might be an additional “programmed” component to aging but this does presumably only apply to a few select species (e.g., some social insects) and is mostly interesting from an academic perspective – but I may be wrong.
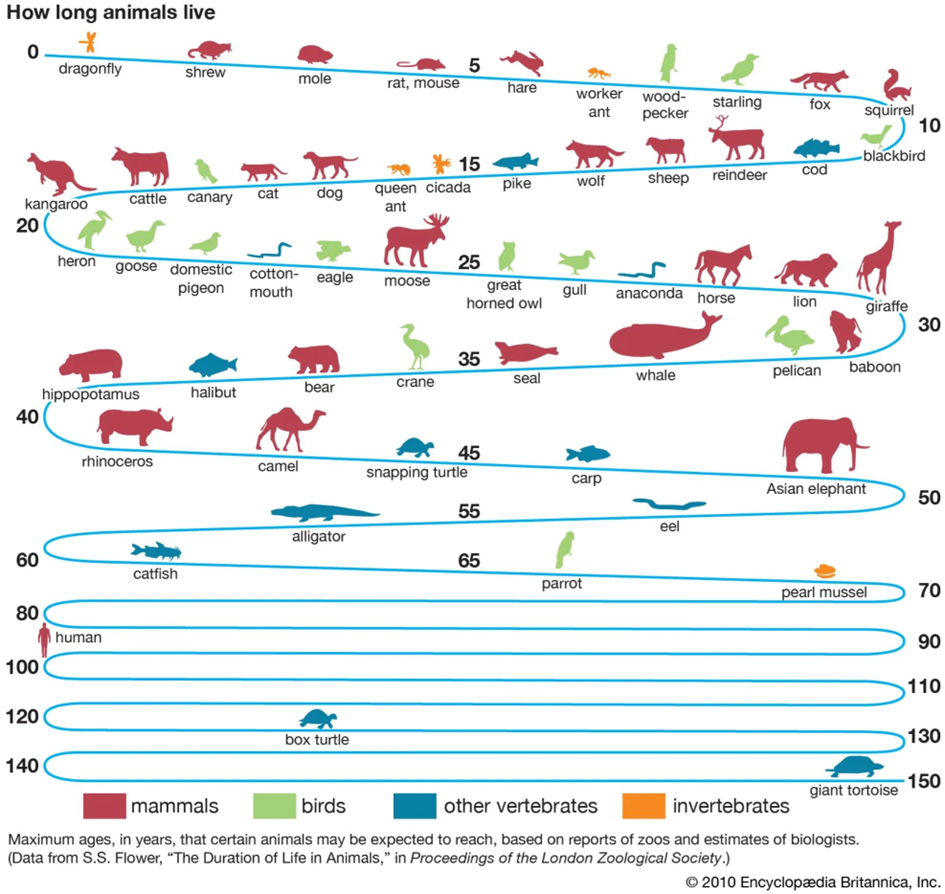
Negligible senescence?
Some animals such as the “immortal jellyfish” (Turritopsis dohrnii) seem to exhibit what is called “negligible senescence”, which means that the animal does not seem to age. Only a small number of species have ever been found for which this is suspected, and their longest-lived individuals ever documented are a few hundred years tops (because they usually die much earlier due to natural causes – limited resources, infectious diseases, and predation).
Theoretically, these organisms might live forever since no law of nature precludes immortality. However, even in perfect laboratory conditions, these organisms might only be able to live for hundreds of years because their senescence is not truly negligible.
For their aging to be truly negligible, the repair machinery for each of the independent processes driving the aging process would have to be 100% infallible, perfect, and bulletproof under all conditions. Something that is, in my opinion, impossible, because just a small progression in any of the cellular drivers of aging would ultimately cause progressive degeneration of the organism (i.e., aging).

Summary
- What is aging? Aging is the deterioration of the organism over time.
- How do animals age? Animals age at the macroscopic level because their cells, tissues, and organs “age” at the microscopic level due to a multitude of interdependent, but independent processes.
- Why do animals age? Aging is spontaneous and accumulating damage by wear and tear is a byproduct of being alive. However, evolution can balance how much energy is invested into repair processes. Negligible senescence is in theory possible if the repair machinery is perfect in all aspects (which is utopian). For a few select species, there might be a programmed component to aging, which is biologically feasible and would also make sense under certain circumstances. However, for most species (including Homo sapiens), this programmed component is (probably) minor.
Sources & further information
- Scientific article: Aging as a Risk Factor for Disease
- Scientific article: The Hallmarks of Aging
- Website: Wikipedia – List of longest-living organisms
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Part III. How to Fight Aging
Two observations raised my interest in longevity science:
- Very similar species sometimes have very different lifespans.
- Genetic experiments (e.g., knocking out the daf2 gene – the receptor for IGF-1) have shown that the speed of aging is quite malleable. In fact, over the last two decades, it became clear that aging is partly governed by a number of “control knobs”, which can be twisted by evolution (or humans).
As explained earlier, the progressive degeneration of an animal (i.e., aging) is a multifactorial issue and no single underlying process is able to fully explain all of it (even though some “influencers” try to make people believe otherwise). Because at least one or more of these aging drivers will relentlessly progress, aging cannot be “cured” but can only be delayed. To delay it, it is ideally attacked from many different angles at the same time.
To effectively fight aging, three unique layers need to be targeted independently. First, cellular health. Second, organ health. Third, organism health.
1. Cellular health
A lot of the aging process happens at the level of individual cells. I presented some of these “cellular drivers” of aging in Part II. I will now differentiate between “endogenous” and “exogenous” drivers.
Endogenous drivers of aging
“Endogenous drivers of aging” are multiple, independent processes that progressively hamper the vitality of single cells. These originate from within the organism (hence “endogenous”), are unavoidable, and would occur and progress even under perfectly sterile laboratory conditions. These include:
- Progressive accumulation of senescent cells
- Stochastic loss of stem cells
- Accumulation of mutations
- Epigenetic changes (especially increased DNA methylation)
- Mitochondrial dysfunction (including mutations of mitochondrial DNA)
- Generation of reactive oxygen species, which then wreak havoc on extracellular and intracellular macromolecules
- Accumulation of endogenous waste products such as lipofuscin or fatty plaques
Exogenous drivers of aging
The “exogenous drivers of aging” originate from outside of the body (hence “exogenous”). They are mostly environmental influences that are theoretically avoidable. These include:
- Ionizing radiation (including Earth’s background radiation)
- Anything causing systemic inflammation (e.g., advanced glycation end products, viral infections, autoimmune issues, etc.)
- Ingestion of undegradable waste (e.g., metals in diet, microplastics, aerial particulate matter, etc.)
- Excess cellular division and mTOR activation due to excess insulin or IGF-1 because of a poor diet and/or poor metabolic health
- Stress-induced excess cortisol leading to increased catabolism and downregulation of repair processes
- Hormone deficiencies leading to lack of anabolism and/or reduced cellular repair
- etc.
The tools & strategies I implement to fight these will be explained in more detail shortly.
2. Organ health
As already explained in Part II, I am a multicellular organism. My organism has a variety of specialized tissues, organs, and organ systems that serve a specific function that cannot be carried out by other cells that are not specialized for this function. Therefore, I need to make sure that no single specialized tissue, organ, or God forbid organ system, degenerates or fails.
There is extensive overlap between cellular health and organ health. The decline of a single organ system is often -but not always- connected to systemic cellular aging, which is driven by the endogenous and exogenous drivers presented above.
In fact, the most common causes of death are not due to systemic aging directly, but due to a degeneration of a single organ system (usually atherosclerosis, cancer, or neurodegeneration), which is often – but not always- driven by the aging process. However, cellular health and organ health can be disconnected.
For example, I could have perfect cellular health but for some reason such as infection, trauma, autoimmune attack, or cancer, etc. my liver could suddenly become compromised. Consequently, metabolism is impaired, toxic waste products accumulate, and plasma nutrients cannot be maintained. It won’t be long before cellular health will deteriorate as well, kicking the various “endogenous drivers” of aging into high gear.
Similar things hold true for other organ systems. If any of the organ systems fail in isolation, even if systemic cellular health were otherwise impeccable, this will cause rapid and massive ripple effects eventually leading to body-wide impairment in cellular health as well, causing the whole organism to degenerate and die.
For example, my grandpa recently died. He was in otherwise good health, but his hip joint was a mess due to years of hard work at his farm. Unfortunately, he was denied hip replacement. Consequently, he had a hard time walking and he started to just lie around in his living room (whereas before he was decently active).
Not even one year later, he died, mostly because his cardiovascular system had deconditioned leading to congestive heart failure. His musculoskeletal system (more specifically, one single joint) had failed him, ultimately leading to his death. It is no coincidence that mortality rises by 1300% in the first year after a hip fracture.
Other examples:
- Maintaining a healthy cardiovascular system: This entails aggressively fighting atherosclerosis (explained in more detail later). Cardiovascular events kill about 50% of people in the industrialized world.
- Maintaining a well-functioning nervous system: This entails preventing neurodegeneration. The nervous system is the major control center for the rest of the body, and without it, nothing else matters.
- Maintaining a healthy kidney, liver, gastrointestinal system, lung, and other organs: This entails the protection of these organs from the usual causes of pathology, including infection, infarction, cancer, injury, toxicity, and autoimmune attack.
3. Organism survival
Lastly, my lifespan can be limited by the premature death of my organism as a whole. Here are a few potential causes:
- major trauma (e.g., leading to blood loss or pulmonary embolism)
- toxicity (e.g., poison, overdose)
- suicide, homicide, or accident
- a large number of diseases

Creating a game plan
As it might be obvious by now, it is almost impossible to completely “cure” aging, as all of the factors above would need to be counteracted or prevented nearly perfectly. However, using the right combination of strategies, the progressive decline of the organism (aging) can be slowed down.

Goal: Delaying aging.
Strategies:
- #1: Promoting cellular health by reducing the endogenous and exogenous drivers of aging
- #2: Maintaining organ health
- #3: Preventing premature death
Tactics: The possible tactics to carry out these strategies are almost limitless. Some are available now and many have yet to be discovered or invented. Unfortunately, we are limited by existing knowledge (e.g., of cellular mechanisms) and available technologies (e.g., pharmaceuticals).
Sources & further information
- Scientific article: Aging and Longevity in the Simplest Animals and the Quest for Immortality
- Scientific article: Clinical Trials Targeting Aging
- Scientific article: Modelling the Molecular Mechanisms of Aging
Part IV: My Longevity Protocol
Aging is the progressive deterioration of an organism over time. This decline happens due to a multitude of different and independent factors, which are all cross-interacting and driving each other in a number of ways.
As a consequence, every single one of my bodily systems slowly but relentlessly degenerates. The cardiovascular system degenerates resulting in atherosclerosis, the bones degenerate resulting in osteoporosis, the joints degenerate resulting in osteoarthritis, the muscles degenerate resulting in sarcopenia, the nervous system degenerates resulting in dementia, the eyes degenerate resulting in presbyopia, the inner ear degenerates resulting in presbycusis, the immune system degenerates resulting in immunosenescence, etc.
If my objective is to slow down functional decline, I need to attack aging from multiple angles simultaneously. Because a healthy diet, sleep, exercise, and stress reduction can only get me so far, I also rely on pharmaceuticals. More fancy methods on the horizon are discussed in Part V: The Exciting Future of Longevity Medicine.
Over the years, I have experimented with many different exercise regimens, diets, hormones, and prescription drugs. At one point, I was taking seven or eight hormones plus about ten prescription drugs at the same time. A good friend once said that self-optimization is a little like porn. People start out with the soft stuff, then move on to the not-so-soft stuff, and gradually end up at the hard stuff.
I started out with supplements but at one point, I was taking over fifteen prescription drugs. After a messed-up plant-medicine experience, I was thrown into a deep existential crisis. I decided to come off everything. Over the course of only a week, I withdrew about a dozen drugs at once. For the first time in 7 years, my body was functioning entirely on its own, which was a deeply spiritual experience.
Since then, I have redesigned my protocol from scratch and have considerably cut down on the number of pharmaceuticals while ramping up my lifestyle efforts.

Because this article is already quite long, in the following sections, I will limit my discussion to the basics while linking to articles in which I cover the topics in much more depth.
Genetics
Genetic factors trump a lot. This holds true for longevity in the same way it holds true for intelligence or athleticism (for more: Biohacking Can’t Beat Genetics).
Many of the supercentenarians (above 110 years of age) made it to this age not because of their lifestyle. Some of them had been drinking or smoking for decades. Their longevity mostly comes down to impeccable genes.
People with two copies of the ApoE2 allele are pretty much immune to Alzheimer’s disease, and people with genetic PCSK9 hypofunction are pretty much immune to cardiovascular disease, the latter of which kills about 50% of the population. People with certain versions of FOXO3 are three times more likely to make it to 100 years.
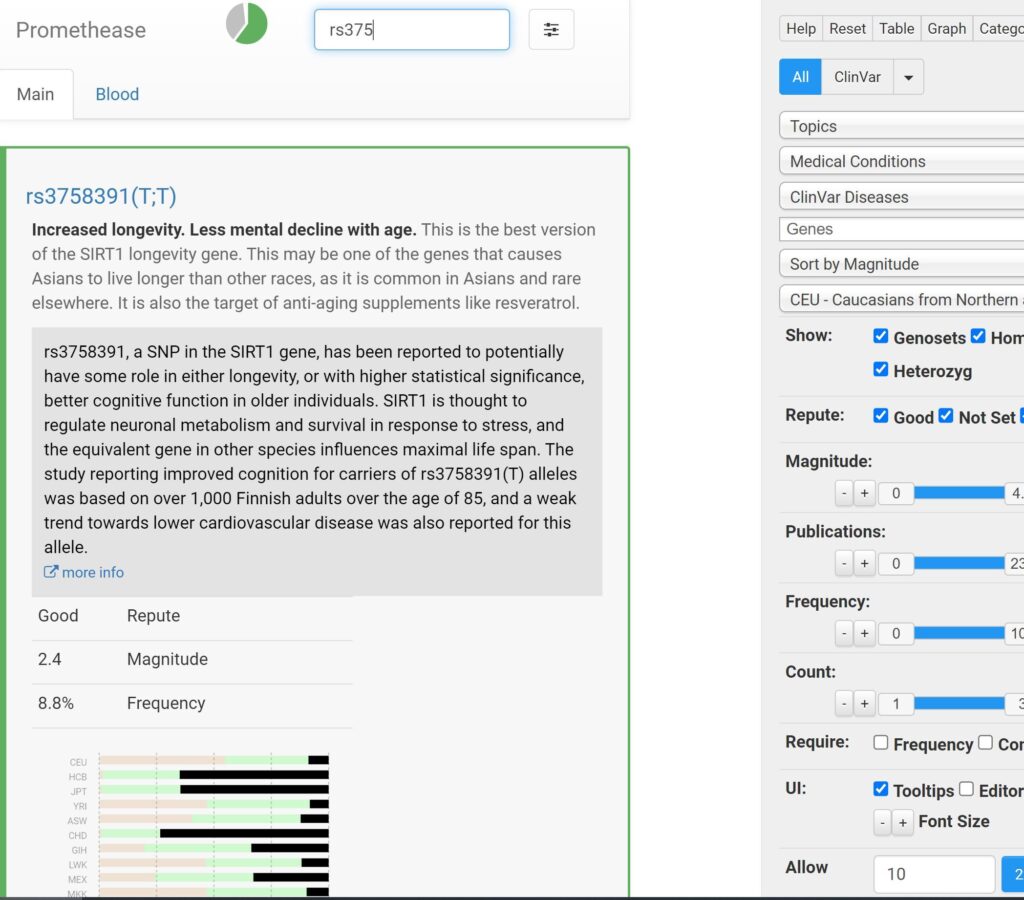
When it comes to longevity genetics, I was quite lucky.
- I have favorable SNPs in a lot of the known longevity genes (e.g., SIRT1, FOXO3, AKT1, p53, CDK2ND).
- Other than a Leiden mutation, I do not carry any genes that are strongly linked with a specific disease (e.g., Lp(a), PCSK9 hypofunction, LDLR, APO E, PPAR subtype polymorphisms, HLA genes associated with autoimmunity).
However, humanity’s knowledge about genetic factors and how they interact with other genetic and non-genetic factors to cause a certain phenotype is still quite limited. Regardless, perhaps the single most important thing I can do to live longer is to have picked the right parents. My grandpa recently died at age 96 without any evidence of dementia, so there is a decent chance that I got some of my favorable longevity genes from him.
I´ll discuss some of the findings of my genetic tests in more detail here: What Genetic Tests Have Told Me About My Longevity
Diet
For a long time, I had been practicing intermittent fasting + a low-carb paleo-keto style diet. Overall, this was presumably overkill and made me feel and function worse.
I have stopped all kinds of fasting (including multi-day fasting), have increased my carbs from 100 to about 200-300g per day, and have considerably upped my caloric intake from 2500 to roughly 3500 kcal per day, which has increased my vitality by a significant degree.
I believe that a lot of the common advice from “health gurus” is detrimental if not dangerous to healthy people – especially if they mess up the context in which this advice is given (namely, to unhealthy people).
I now believe that if I cut out most processed food and sugar, get an adequate caloric intake (which is much higher than I was led to believe), and titrate my carbs to leanness and activity levels, I already get 80% or so of what is maximally attainable through dietary intervention. Therefore, given these things are met, I now believe diet to be a low-leverage lever to pull if compared to sleep, exercise, or drugs.
I occasionally wear a blood glucose monitor and I try to limit glucose excursions.

I discuss my diet in much more detail here.
Exercise
Even though the section on exercise is short, for several reasons, daily exercise is one of the best things (if not the best thing) I can do to improve my longevity. I do about 1 hour of exercise per day with usually one rest day per week. I generally alternate between resistance training and cardiovascular exercise.
More specifically, I do two to three 1 hour sessions of zone 2 exercise per week, each of which I usually end with 10 minutes of high-intensity training. To maintain strength and muscle mass, I do a full-body workout twice per week. In terms of mobility, I do daily stretches, including my psoas major, quads, and pectoralis muscles, and I strengthen my glute medius and tibialis anterior.
In addition to strategic exercise, I aim to also get in a lot of casual movement throughout the day. For example, I love going for walks and I also interrupt long blocks of sitting with a 2-minute kettlebell exercise every two hours.
I discuss my exercise regimen in much more technical detail here.

Sleep
I put a prime on behavioral tactics to optimize my sleep. These include going to bed at the same time, going to bed earlier than later, sleeping in a pitch-black dark and quiet environment, having a cooling blanket, and a handful of supplements.
I discuss my approach to sleep enhancement in much more detail here.

Supplements
Out of all of the points on this list, supplements are most likely the lowest value item. I take a lot of supplements. These include 1g salt, 2g EPA/DHA, 600mg magnesium, 15mg zinc, 3.000 IU vitamin D, 2400mg phosphatidylcholine, 500mg L-acetyl-carnitine, 1g beta-alanine, 300mg NAC, 1g TMG, 250mg VitC, 1mg L-methyl folate, 45mcg VitK2, 5g glycine, 500mg taurine, 60mg CoQ10, 500mg inositol, and occasionally a low-dose vitamin B-complex.
I also take astaxanthin, the only proven longevity supplement (discussed here).
Many of these supplements are probably a waste. Unfortunately, I do not know which ones.

I avoid plant/herbal/fungal supplements (e.g., ashwagandha, rhodiola, curcumin, etc.) because I believe that whatever good can be got from them can also be gotten from pharmaceutical drugs, without the countless off-target effects, messing up the Cytochrome P450 enzyme system, and without playing roulette with manufacturers. Furthermore, I believe the “anti-aging”-supplements resveratrol and NAD/NMN are close to worthless for anti-aging purposes.
I discuss the supplements I take, and why I take them, in more detail here.
Mental health
From a longevity perspective, mental health underlies much of what causes disease and death, in part because mental health influences lifestyle and behaviors. I believe that “baseline vitality” is one of the most important underlying factors determining my mental health.
For a list of articles on mental health, see here.
Metabolic drugs
Metabolic health is the cornerstone of longevity. Over the last couple of years, I have experimented with a host of metabolic drugs, including lipid-lowering agents, antidiabetic drugs, antihypertensives, uric acid-lowering drugs, and vasodilators. However, few of these have stood the test of time and I currently only take a very low dose of allopurinol. I recently stopped the low dose of semaglutide & metreleptin because I am currently trying to gain weight.
I describe my experience with these drugs in more detail here.

Hormones
A proper hormonal balance is essential to disease prevention, health, longevity, and quality of life. However, replacing hormones is a tricky thing to do. Turn one gear and many other gears turn as well. At the moment, the hormones I supplement with include
- Vitamin D
- A low dose of hCG to increase my testosterone and estradiol levels (but without my HTPA being shut down). I combine this with a low dose of finasteride, which seems to affect aging favorably. For more: My TRT Lite Protocol
I discuss my experience with hormones here. I discuss the topic of hormones & longevity in more detail here.

Downregulating the mTOR pathway
Some of the few interventions that seem to offer robust longevity benefits are rapamycin, caloric restriction (CR), and some degree of fasting. They have a lot of overlapping mechanisms and benefits.
Most importantly, they all suppress mTOR. Simplistically speaking, cells are in a “growth mode” when mTOR is highly active and in a “conserve-resources mode” when the mTOR pathway is little active.
mTOR is crucial for the growth and development of an organism to reach sexual maturity. However, usually, organisms die long before aging kills them, which means that evolution never bothered with reducing the activity of growth pathways after sexual maturity is reached.
Many experiments have shown that mTOR is one of the few “control knobs” of aging and elevated mTOR is at the center of many age-related diseases. mTOR inhibitors are a (potential) chemical fix for a problem evolution never bothered to address.
Pharmacologically reducing the activity of the mTOR pathway mimics the positive life-extension and health-extension effects of caloric restriction and combats many aspects of the aging process.
In laboratory animals, rapamycin slows cellular senescence, improves metabolic health, and helps prevent atherosclerosis, cancer, and neurodegeneration. Furthermore, there is evidence that mTOR inhibition slows down the rate of degeneration of the brain, heart, bones, kidney, liver, skin, adrenal glands, ovaries, stem cells, and pretty much every other tissue and organ. Furthermore, rapamycin seems to be pretty good at reducing age-related sterile inflammation.
I have been taking weekly doses of rapamycin for a little over 5 years. I started out with higher doses (6-7mg/week), though over time I have decreased my dosage to 4mg once per week while taking a 3-month break during the winter months.
Initially, I had some annoying canker sores, some hyperglycemia, and some pitting edema in my legs. After a couple of months, I have not noticed anything, either good or bad. Sometimes I stop taking rapamycin during the winter months because I seem to catch more viral infections on it – in fact, off rapamycin, I barely ever catch a viral infection.
One could argue that, for me, being in my late twenties, the risk-reward ratio does not make sense as in the next 10-20 years we will have a lot more info on the risks of rapamycin. And I partially agree. However, towards the end of their thirties, many people report worse recovery, flexibility, greying hair, changes in body composition, and metabolic issues, among other things.
My thirties are my last “youthful” decade and if I can prolong my health and vitality by a bit, I am willing to take the (small?) risks associated with mTOR inhibitors. In my opinion and as I currently see it, taking rapamycin has fewer risks and downsides than not taking it – but I could be wrong. I am continuously monitoring the space and I am open to changing my mind.
I describe my experience with rapamycin in more detail here.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Senolytics
As I age, more and more of my cells become senescent. Senescent cells can be compared to “zombie cells”. These cells are dysfunctional and are incapable of performing their normal tasks. However, they do take up space, which prevents stem cells and healthy cells from repopulating their niches with healthy cells.
Furthermore, senescent cells also directly hamper tissue health by secreting proinflammatory mediators (they take on a so-called “senescence-associated secretory phenotype” – SASP), including proteases, cytokines, and myokines.
In theory, if senescent cells are cleared, two things happen:
- Their cellular niches are now vacant and can be filled by new, healthy cells (derived from resident stem cells or healthy surrounding cells).
- They stop secreting proinflammatory molecules.
While rapamycin slows geroconversion (meaning that it can delay the time until cells become senescent), senolytics clear cells that have already become senescent. I have done a couple of “hit&run” cycles with senolytic drugs aimed at clearing out some of these cells.
In animal models, clearing senescent cells has been shown to ameliorate a plethora of age-related pathologies including atherosclerosis, osteoporosis, osteoarthritis, sarcopenia, hepatic steatosis, cataract formation, obesity-induced metabolic dysfunction, and possibly neurodegeneration.
To clear some of my senescent cells, I used a combination of dasatinib and quercetin, the latter of which is probably of limited utility. Dasatinib is the only senolytic clinically available for which extensive experience is available with both humans and non-human animals.
Thus far, I have done six “hit & run” cycles with dasatinib (140mg/d) & quercetin (2g/d). My last cycle was over 2 years ago and I do not plan to do a new cycle anytime soon until I have more data.
I describe my personal experience with senolytics in more detail here.
Why I do not use peptides
I discuss my personal experience with peptides and why I am literally scared of using them in more detail here.
In-depth articles
I discuss all of the points I have just discussed in much more technical & medical detail here:
- My General Longevity Framework
- My Protocol For Fighting Heart Disease
- Preventing Cancer
- Combating Dementia
- Hormone Optimization for Longevity
- Optimizing Metabolic Health
- Reducing the Activity of the mTOR Pathway
- Eliminating Senescent Cells
- Lowering Inflammation
- Maintaining Healthy Bones & Muscles
- Optimizing Organ Health
- Avoiding Toxins & Minimizing Infections
- Optimizing Mental Health
- What Genetic Tests Told Me About My Longevity
- Side Effects of My Longevity Protocol
Cost
About 800$ per month (including food). As I was still on metreleptin, the cost was much more.
Effort
It might seem that adhering to my regimen takes a lot of effort. It does not.
- I prepare pillboxes about once per month (1h), and take my supps & drugs two times per day (morning; before bed) with a glass of water.
- Injecting HCG (roughly 1 minute per day)
- Exercise (including my way there and back) (1.5 hours per day)
- Preparing foods (20-30min per day) – I am all for convenience (e.g., Kefir+ olive oil)
- Extensive blood work every 3 months
Developing and fine-tuning everything took me years to do, but once it had been set up, it now takes me an average 5 minutes per day (excluding exercise and preparing food).

How did this start?
Due to being too much into “fitness”, I developed stubborn hormone problems at the age of 21, followed by medically supervised hormone replacement therapy. After experiencing the extent to which exogenous intervention was able to transform my outer and inner life, I started to go down the rabbit hole of biological self-optimization.
Slowly, one at a time, and with a deep understanding of how things work, I experimented with a lot of different things. I kept about 5% of the things I experimented with.
Am I worried about long-term consequences?
For some of my interventions, I do not have the proper long-term data I would like to have (i.e., in the context of a healthy person). So yes, I am worried about the long-term consequences of my actions. However, I am equally if not more worried about the long-term consequences of inaction.
(Potential) Benefits
All of the biomarkers I test for are great, though most of them have also been great before. I look young for my age, I feel well, I am athletic, I have good energy levels, and I can work in a focused way for long hours. But so are many others my age. Thus far, I have not seen any obvious benefits of my regimen. However, encouragingly, I have also not experienced any obvious downsides over the course of over half a decade.
My protocol is probably far from sufficient to meaningfully counter the aging process. Rather than extending maximum lifespan, it may have a much stronger effect on my health span, hopefully allowing me to live in a state of reasonable vitality until my time in this universe has run out.
Goals
My objective is to strike a decent balance between longevity and vitality (as unfortunately, longevity and vitality are sometimes at odds – for example, caloric restriction). I personally do not care about maximum life span extension as this comes with quality of life tradeoffs I am not willing to make. Said in other words, my objective is to live well for a long time (health span).
If I had to choose between longevity and vitality, I would choose vitality without a second thought. Fortunately, these two are not necessarily mutually exclusive. Longevity is not only about prolonging health span, but also about being healthy and feeling good in the here and now because improving metabolic, cellular, and tissue function presumably also benefits me in how I feel and function in my everyday life.
Risks
Many people perceive what I do to be aggressive, dangerous, detrimental, risky, and stupid. However, I believe that most of the things I do are much less aggressive, dangerous, detrimental, risky, or stupid compared to e.g., alcoholism, tobacco abuse, or obesity, which affect about 50% of the population.
In my opinion, the major difference between these things and what I do is the so-called “familiarity principle”: The more we are exposed to something (e.g. alcohol, cigarettes, obesity), the more familiar we are with it – and the more familiar we are with something, the more normal and safe it seems. However, my cells could not care less about arbitrary culture-bound familiarity.
Even though some of this is uncharted territory (at least it was when I first started doing it five years ago), I believe that the risks of my current regimen are quite low given the currently available clinical and experimental data. The riskiest thing I did was most likely CrossFit.
Furthermore, I continuously monitor a number of things, I do a full-body MRI every three years, and I have ready access to a lot of great doctors. People who are doing anything similar without extensive self- and other-monitoring, particularly if said people do not have a medical background, are likely heading for catastrophe.
Side effects
I have been on this or a similar protocol for about five years. No major side effects as of yet. I´ll discuss some minor side effects and roadblocks in more detail here: Side Effects of My Longevity Protocol
Philosophy
I briefly describe my philosophy, and why I do everything I do, here.
Part V: Ranking of Longevity Interventions
In this section, I try to rank the most effective longevity strategies. Since controlled experiments are lacking, this ranking is based on a combination of available data, first-principle reasoning, and gut feeling. It is more likely than not that I am wrong on a number of things.

Very high value
All of the things in this category I deem extremely valuable for longevity (healthspan & lifespan). At first I will discuss the basics (diet, sleep, exercise), and then I will discuss more aggressive interventions that are presumably of a very favorable risk-reward ratio.
Mental health, connection, purpose
Even though this sounds cliché, when mental or emotional health is bad, all of the other things do not matter. Furthermore, with bad mental health, it is just too easy to slip up in the lifestyle department, which is crucial for all things longevity-related.
Having meaningful relationships and a sense of purpose are both linked to longer health and lifespan even though the arrow of causality is not clear because more vital individuals are more likely to have better relationships and more likely to have a sense of purpose.
I discuss these in more detail here.
Exercise
Getting an adequate amount of exercise is essential for a number of things that are intricately linked with the aging process.
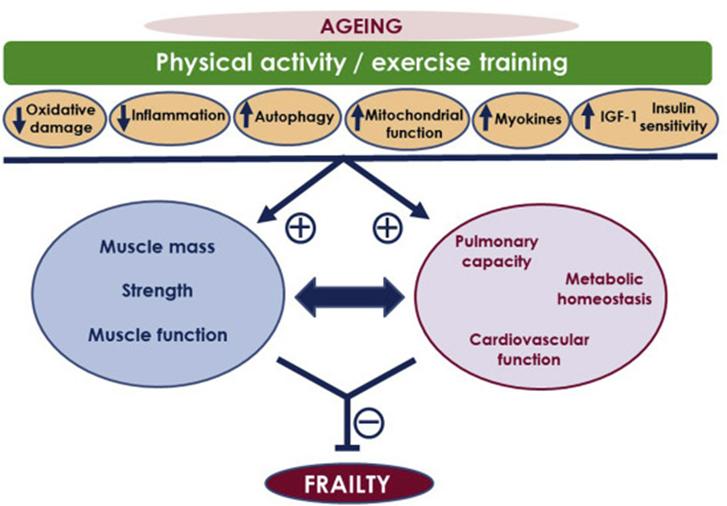
Four different kinds of exercise have independent benefits:
- Longish sessions of aerobic exercise (so-called “zone II” exercise) build aerobic endurance, which is the “base” of everything else. Training aerobic endurance has highly beneficial effects on metabolic health and brain health through a plethora of cross-interacting mechanisms.
- High-intensity cardiovascular exercise (so-called “VO2 max training”) induces a large number of molecular changes that favor a slow-aging phenotype (e.g., increased expression of uncoupling proteins). VO2 max (maximum rate of oxygen consumption attainable during physical exertion) is highly correlated with longevity.
- Resistance exercise, which builds strength and muscle mass (or at least maintains them), is important for metabolic health and structural integrity. Similar to VO2 max, strength is highly correlated with health span and lifespan.
- Mobility exercises train proprioception and also keep muscles and joints flexible, all of which are important for maintaining functionality and preventing falls in old age, which usually lead to a rapid decline in health.
Next to strategic exercise, it seems optimal to do a lot of casual movement (or strategic “exercise snacks”) throughout the day (e.g., talking walks).
Out of all the myriad beneficial effects of exercise (e.g., metabolic health, mitochondrial health, musculoskeletal integrity), I find the beneficial effects on both brain function and brain health the most valuable, given that my nervous system is the basis of my consciousness. In fact, exercise is probably among the most effective ways to counteract neurocognitive decline.
I discuss my exercise protocol in more detail here: My Physical Fitness Protocol
Sleep
Sleep is not only important for short-term brain function (attention, executive functions, memory, willpower) but also for long-term brain health. Furthermore, sleep deprivation worsens metabolic health and elevates levels of cortisol – both of which drive the aging process. Also, in a sleep-deprived state, metacognition and willpower are usually so poor that leading a healthy lifestyle becomes awfully hard.

I discuss my approach to sleep optimization in more detail here: How I Biohack My Sleep.
Diet
Going from a “bad” to a “decent” diet is presumably of very high value in terms of health and longevity. Most important levers:
- cutting out crap (i.e., highly processed food)
- titrating carbohydrate intake to leanness and activity levels
- getting an adequate caloric intake (too little is just as bad as too much)
- avoiding large amounts of sucrose and fructose – especially for metabolically unhealthy individuals
- getting enough micronutrients
However, I believe that going from a “good” diet to a “perfect” diet is of low value.
I discuss my own diet in more detail here: My Approach to Diet
Losing body fat
Having a higher body fat is somewhat linearly correlated with low-level inflammation and poor metabolic health. For most people, it is almost impossible to be “healthy” if their body fat is above a certain percentage. I consider this to be above 25% for males and above 35% body fat for females (though, individuals vary). I discuss body fat in more detail in the section on diet.
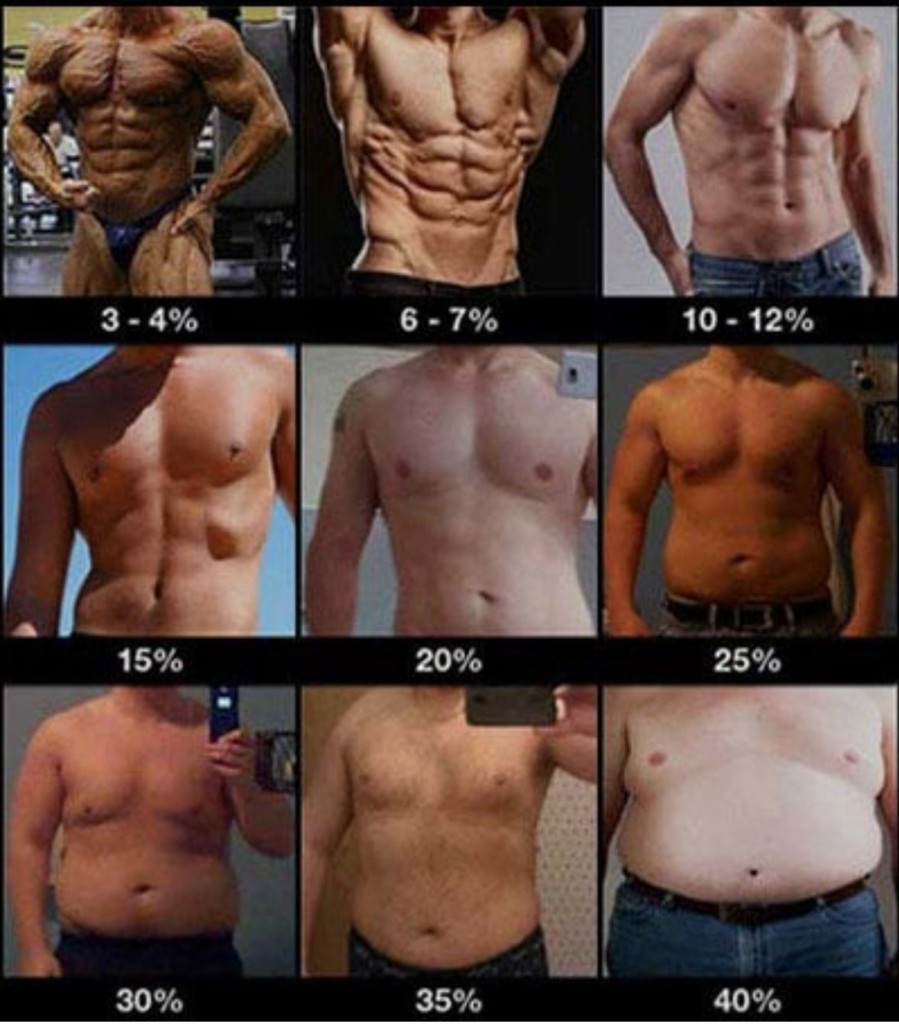
I discuss weight loss in more detail here: Why Weight Loss is Not About Calories In vs. Calories Out
Rapamycin
Many researchers speculate that rapamycin is presumably among the most potent geroprotective molecules currently available to mankind.
Cells can be either in “repair mode” or “growth mode” and the balance of these two modes is partially decided by a protein complex called mTOR. By inhibiting mTOR, rapamycin is a starvation mimetic that chemically mimics the positive life-extension and health-extension effects of caloric restriction.
Rapamycin, and other rapalogs, are thought to prevent (or at least delay) a number of age-related diseases, including atherosclerosis, obesity, neurodegeneration, osteoporosis, and retinopathy. Furthermore, rapamycin is thought to rejuvenate stem cells, reduce immunosenescence, and improve metabolic health. Rapamycin presumably also has potent and widespread anti-cancer benefits.
I discuss rapamycin, and my experience with it, in more detail here: My Experience With Rapamycin
Hormone optimization
Hormones are crucially important for the structure and function of organs, tissues, and mind and whenever one of the “master control” hormones is off (thyroid, sex hormones, cortisol, GH/IGF1, leptin), many aspects of the body & mind start to suffer. Furthermore, if one of the major hormones is off, everything else becomes a lot harder as they have a major effect on well-being, energy levels, mood, motivation, and general health.

Hormone deficiencies are also (causally) related to a number of age-related conditions, including obesity, diabetes, hypertension, depression, chronic fatigue, osteoporosis, and neurodegeneration.
In my opinion, replacing what is missing is just as unnatural as it is to live beyond the age of 40 in the first place. Furthermore, hormone replacement therapy is presumably also “better” than the pharmaceutical approaches of the current sick care system for taking a separate drug for each individual sign and symptom.
Hormones are a huge topic. I discuss hormones in more detail here: The Life-changing Power of Hormones
Metabolic drugs
Metabolic health is at the center of chronic disease and aging. Metabolic enhancers are powerful drugs to change certain aspects of metabolism, including but not limited to insulin regulation. While these are not “necessary”, they can be highly helpful (particularly for metabolically unhealthy individuals). Obviously, the more metabolically sick someone is, the more powerful these drugs tend to be.
I discuss metabolic drugs, including GLP-1 agonists, SGLT-2 inhibitors, metformin, acarbose, and allopurinol in more detail here: My Experience With Metabolic Drugs
I particularly want to single out SGLT-2 inhibitors. SGLT-2 inhibitors are among the most organ-protective substances known to mankind. In fact, they are frequently prescribed for kidney or heart failure even if the patient is not diabetic and they are thought to prolong organ health and function for quite a bit. For example, they are known to delay the time to dialysis for kidney disease patients by over a decade. Similar things hold for heart failure.
They are also thought to promote the health and function of a number of other organs, including the brain. Furthermore, SGLT-2 inhibitors reduce cancer incidence. In the intervention testing program, canagliflozin prolonged the lifespan of genetically heterogeneous male mice by a whopping 14%.
Getting blood pressure in order
High blood pressure is a silent killer: It drives cardiovascular disease (including its consequences), neurodegenerative disease, and kidney disease. Out of all of the Framingham factors (hypertension, smoking, hyperglycemia, dyslipidemia, obesity), hypertension is the deadliest – estimated to be responsible for about 8 Mio. deaths per year (15% of all deaths).
For every 10mmHg reduction in RR, the risk for cardiovascular events drops by 45% (data derived from Mendelian randomization).
If my blood pressure were high, I would use the angiotensin-receptor-blocker (ARB) telmisartan, which also has a favorable effect on metabolic health due to its affinity for the PPAR delta receptor (a metabolic master control switch in skeletal muscle).
Reducing blood lipids
Lipoprotein particles are the causative agent of atherosclerosis (“necessary but not sufficient”). The most relevant lipoprotein is LDL, which is best measured by an ApoB blood test. Cardiovascular disease kills about 50% of people in the modern world. Furthermore, a compromised cardiovascular system leads to poor organ health resulting in kidney disease, dementia, erectile dysfunction, and hypoperfusion of a host of bodily tissues.
If one desires to live a long and healthy life, lipid levels must be in order. For every 40mg/dl reduction in LDL, the risk of CV events drops by 55% (Mendelian randomization data). Similarly, a reduction of 25mg/dl LDL + a reduction of 5mmHg RR reduces the risk by 55% as well.
Fortunately, there are a large number of great lipid-lowering agents, which in most cases, are much superior to lifestyle changes. Most people respond well to a very low dose of rosuvastatin (2.5-5mg) in combination with ezetimibe. This combination is probably the best bang for the buck. Other lipid-lowering agents worth mentioning are bempedoic acid and PCSK9 inhibitors, both of which are quite powerful, have few to no adverse effects, but are expensive.
Furthermore, lp(a) levels highly correlate with heart disease and is the most common cause for early heart attacks. As of now there are no drugs other than PCSK9 inhibitors that can lower lp(a) significantly. Fortunately, a host of various lp(a)-lowering therapies are currently in Phase III of clinical development, including a once-in-a-lifetime CRISPR gene therapy.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
High value
While not as valuable as the items in the category above, in my opinion, the following things are of high value for improving health span and lifespan.
Getting a genetic test
Getting a genetic test done can be quite helpful because it allows for early intervention in case of known deleterious genes. Most importantly, these genes include the APO E phenotype (discussed in more detail here), which is highly associated with the incidence of dementia.
I discuss the findings and practical takeaways of my own genetic test in more detail here: What Genetic Tests Told Me About My Longevity

Senolytics
I tentatively put senolytics in the high-value category, however, I am quite unsure. Could be very high value, could be low value, could even be detrimental. Regardless, as of now, it seems that intermittently clearing an aging organism of senescent cells (likely) has a variety of benefits for the overall functioning of the organism, especially at older ages.
Simplistically speaking, senescent cells are dysfunctional cells that take up valuable space and promote tissue-level inflammation. Possibly, they also induce other cells to become senescent.
Several classes of such senolytic drugs have been identified, including BCL protein family inhibitors (regulators of apoptosis), kinase inhibitors (growth factors), heat shock protein 90 inhibitors, and inhibitors of the p53–MDM2 interaction.
Dasatinib, a tyrosine receptor kinase inhibitor, is the most researched senolytic agent currently available – however, there will be more and different ones in the coming years. I discuss my experience with senolytic agents in more detail here: My Experience With Senolytic Drugs
Monitoring health markers
Monitoring metabolic health markers gives real-time feedback on what is or isn’t working and provides a way to know when to intervene accordingly. I personally monitor a set of hematological, hepatic, renal, metabolic, immune, and hormonal parameters by doing extensive blood work four times per year. I also keep an eye on my vital signs and measure my blood glucose via a CGM. I discuss the specific parameters I test for in more detail here.
Some nutritional supplements
The ones I routinely recommend to family & friends include fish oil, magnesium, L-methyl folate, vitamin D, glycine, and potentially an injectable vitamin B12.
I discuss these supplements in more detail here: Supplements I Take
Cancer monitoring
Depending on one’s age, cancer monitoring can be of quite high value (e.g., colonoscopy, prostate screening, mammography). I am quite young. Therefore, all the cancer monitoring I currently do (testing for a set of plasmatic tumor markers once per year + a full-body MRI every couple of years) is presumably of low value.
Given that the world as we know it still exists in 20 years, I suspect that liquid biopsies, which test for free-floating cancerous DNA and potentially metabolomic and proteomic profiles, will hopefully be widely available and are going to be routinely done via a simple blood test.
Avoiding cytomegaly virus (CMV)
The cytomegaly virus (a certain type of herpes virus) is poorly coevolved with humans. In people who are infected (depending on the geographic location about 20-50%), it is continuously trying to break out and it is estimated that about 30-50% of the human adaptive immune system is busy fighting CMV in their later years. This constant immune activity targeted at CMV accelerates immunosenescence and causes low-level inflammation, presumably shortening the lifespan & health span by a couple of years. Furthermore, CMV (and other herpes viruses) are associated with neurodegeneration and dementia.
I am still negative for the cytomegaly herpes virus (CMV), and I want to keep it that way. Before dates, I take 1g of valacyclovir (in case we kiss or have sex) which may offer some (slight) protection (and definitely offers protection against HSV-II, which is associated with dementia).
Interestingly, rapamycin is also known to reduce the risk of infection with CMV because it prevents clonal expansion of infected cells. Hopefully, humanity has a safe vaccine available soon because I am eager to get it.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Medium value
In my opinion, the things below are of medium value for longevity and they may be able to move the needle slightly.
Supplements
Most supplements are presumably of little value. Other than the ones mentioned above, the ones that may have some value for prolonging health span include creatine, beta-alanine, taurine, and n-acetyl-cysteine. I personally take a lot of supplements, and I am quite sure that many, if not most, of them are a waste of money. Unfortunately, I do not know which ones. I discuss the supplements I take, and why I take them, in detail here: Supplements I Take And Why I Take Them
Prevention-oriented drugs
It is hard to say what “prevention-oriented” drugs are because they depend on the individuals. I personally have experimented with a couple of them but the only one I still take is allopurinol because my levels of uric acid are naturally quite high, which is associated with hypertension, insulin resistance, and low-level inflammation.
I specifically decided against using aspirin (prolongation of bleeding time), statins (my ApoB is naturally below the reference range), and PDE5 inhibitors (risk for tinnitus and hearing impairment).
Fasting
Fasting is associated with a lot of health benefits. Fasting may be particularly helpful for metabolically ill individuals and may be a great way to become metabolically healthy. For me personally, though, fasting has presumably done more harm than good. I discuss this in more detail here: Why I Stopped Intermittent Fasting and here: How Multi-day Fasting Has Harmed Me

Anti-herpes virus agents
Most humans are infected with two to five herpes viruses, which are as of yet unclearable from the human body because they are retroviruses that reverse-transcribe (integrate) into the genome. Unfortunately, as discussed in the case of CMV above, many of these viruses are on a continuous quest to “break out” of their latent phase. Being infected is associated with a host of conditions, including progressive neurodegeneration.
For this reason, a good friend uses 500mg of valacyclovir daily (and plans on being on it indefinitely) aiming to keep his load of herpes viruses low and to prevent constant reinfection of yet uninfected cells. Whether the upsides of taking valacyclovir (which is presumably the safest herpes antiviral but unfortunately also the least specific for CMV) outweigh the risks is currently unknown, though according to the currently available literature valacyclovir’s safety profile seems to be quite good.
Heat exposure (sauna)
Sauna use mimics moderate physical activity and benefits include an improvement in blood flow, heart rate variability, blood pressure, other aspects of cardiovascular health, and possibly a reduction of all-cause mortality. Sauna use also leads to an upregulation in heat shock proteins, though the benefits of this are debated.
In my opinion, most of the benefits (and more) can likely also be got from aerobic exercise and therefore sauna use has an opportunity cost associated with it (namely, the time spent doing aerobic exercise). I tentatively included it in the ”medium value” category because I am unsure about the value of intermittently increasing heat-shock protein expression. I discuss heat & cold exposure in more detail here.
Antioxidants
I am still not sure how valuable antioxidants are. They are great on paper but probably not overly valuable in real life (and possibly even detrimental if overdone) because firstly, cellular redox status is highly regulated, and secondly, ROS leak is involved in a number of physiological cellular signaling mechanisms.
I personally take a combination of low doses of different antioxidants, including coenzyme Q10 (60mg), vitamin C (250mg), alpha-lipoic acid (100mg), and n-acetyl cysteine (300mg). I am not sure about the benefits vs. harms of this, though my intuition is that at the doses listed, these supplements are quite safe to take indefinitely. I discuss the science behind these antioxidants in more detail here.
Of note, asteaxanthin, a potent antioxidant accounting for the color of flamingos and salmon, is the only supplement that ever passed the ITP, extending the lifespan of genetically heterogenous mice by roughly 10%.
MAO-B inhibition
Compared to adults, children appear hypomanic at baseline. They are emotional, have great energy, are curious, and act impulsively. This is thought to be in part due to their high dopaminergic tone. As they get older, they get less curious and enthusiastic. This may not be exclusively due to them being tortured by archaic education systems, but may also be in part because dopamine levels progressively decline.
In fact, of the about 85 billion or so neurons, only a mere 400-500 thousand neurons manufacture dopamine (the actual count varies between individuals), and approximately 5-10% of these neurons are lost per decade.
Dopamine is critical to approach behaviors of all kinds and to the capacity to switch from one behavior to another. Dopamine is one of the primary (biological) determinants of how excited someone is, how motivated, and how ready one is to push through things to get what he wants.
Catecholaminergic neurons (neurons that produce dopamine and noradrenaline) are among the first to degenerate -mainly because of hydroxyl radicals (toxic oxidative byproducts) produced by the MAO-B metabolism of dopamine. This progressive and relentless die-off can be slowed drastically by MAO-B inhibition. MAO-B inhibitors are drugs used for Parkinson disease but they have been found to be neuroprotective in healthy people as well. Therefore, once I hit 40 I plan on taking a very low dose of rasagiline, an irreversible inhibitor of MAO-B.
I discuss dopamine in more detail here: An Introduction to Neurotransmitters

Furthermore, the heightened levels of dopamine also help with willpower and drive, both of which are essential for leading a healthy lifestyle. I discuss my experience with rasagiline in more detail here.
Teriparatide
Teriparatide is a synthetic analog of parathyroid hormone (PTH), which is important for regulating plasma calcium levels and bone metabolism. Continuously high levels of PTH are catabolic to bone but intermittently high levels (e.g., given once daily), PTH has potent osteoanabolic effects. In fact, PTH-analogs are the most potent bone-building molecules currently available.
Given that bone mass degenerates with age, often leading to fractures that eventually result in inactivity and sarcopenia, it makes sense to boost bone mass using teriparatide at least once after a certain age (e.g., 50 years).
I personally used teriparatide after I dislocated my left shoulder during ice skating. It turns out that teriparatide has chondroregenerative properties as well and seems to counteract the development of posttraumatic arthritis. Whether it worked or not is hard to say but it surely did not hurt. After getting Bankart repair (labral surgery) I continued to use teriparatide to help with labral reinsertion.
17-alpha estradiol
17-alpha estradiol is the natural optical isomer of 17-beta-estradiol (the major form of human estrogen). 17-alpha-estradiol is less active than the beta version, displaying 100-fold lower estrogenic activity at the estrogen receptor beta (ERb) and alpha (ERa), therefore being far less feminizing. However, it seems to activate the ER-X receptor with a greater potency than 17-beta estradiol. The ER-X receptor is highly expressed in the human brain.
In the intervention testing program (ITP), 17-a-estradiol increased the lifespan of genetically heterogenous male mice by 12%, which is fascinating even though unexpected. Interestingly, it does not work if the male mice are castrated and neither does it work in female mice.
Current approaches to regenerative medicine
No matter what I do, aging will take its toll. Currently, there are few useful regenerative medicine technologies available and currently used interventions are often more hype than science. The things that are available now, such as stem cell injections or platelet-rich plasma therapies for healing injuries, are probably of only medium value.
However, there are various very exciting technologies heading our way that may one day be of very high value. I briefly touch on what is coming here: The Exciting Future of Longevity Medicine
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Low value
The following items might have some decent value, though, in my opinion, less than the things mentioned above.
Metal chelating agents
Like antioxidants, I am unsure about the usefulness of (purported) metal chelators such as alpha-lipoic acid, taurine, N-acetylcysteine, and beta-alanine. As of now, I feel more comfortable taking them than not taking them.
I personally would not dare doing more aggressive metal-chelating treatments (e.g., EDTA) because of the risk of redistributing some of the heavy metals to the brain as they are liberated from other organs, particularly bones.
Avoidance of environmental toxins
I believe that for me, a non-smoker living in a central European country, the value of going out of my way to avoid environmental toxins is fairly low because, as far as I can tell, the EU does quite a good regulatory job in that regard. I am unsure of the potential negative consequences of glyphosate and other pesticides but they are probably far less of a concern than what the media makes them out to be.

Obviously, if toxin exposure were an issue (e.g., bad air quality, bad water quality, smoking, lead exposure, etc.), getting this straightened out would be of high value.
Minimizing bacterial infections
I believe that trying to avoid bacterial infections by excessive sterility does more harm than good (in a non-hospital setting). I discuss this in more detail here: My Protocol for Avoiding Toxins and Minimizing Infections.
Cold exposure
While cold exposure increases catecholamine levels (and is therefore a great coffee replacement that indirectly improves sleep) and potentially activates brown fat tissue to a relatively minor degree, I find the value of cold exposure in terms of longevity negligible (especially compared to the other things on the list).
In my opinion, the greatest value of cold exposure in terms of longevity is that it helps to build willpower, which is crucial to everything else. I discuss the long-term consequences of having been a cold shower addict in more detail here: Long-term Side Effects From My Addiction to Cold Showers
Peptides
It seems that nowadays a lot of people are into peptides such as TB500, BCP157, dihexa, cerebrolysin, epitalon, growth hormone secretagogues, etc. While some of them might be safe and might have benefits, we do not know which ones.
While they may sound “cool”, I personally believe that most of them are close to worthless and on the placebo level. However, they make an easy sell to people who do not have access to powerful evidence-based molecules (i.e., pharmaceuticals).
Regardless of whether they work or not, when it comes to peptides, there is little to no human safety data (especially on long-term administration). Furthermore, most peptides are usually manufactured by non-regulated manufacturers, and “white powders from the internet” are suspicious by default, especially if they are intended for injection.
Anecdote time. Once I sprained both of my ankles at the same time. I decided to inject TB500 and BCP157, which are two peptides commonly used for “healing like wolverine”. I bought them from a “reputable” online seller – whatever that means. However, “for science”, I injected them into only one of the injured ankles for two weeks. I have not noticed any difference in recovery time whatsoever.
Resveratrol & NAD+/NMN
If there was a “no-value” category, these would probably belong there. While both of these are most likely low-risk (with the possible exception of NAD-precursors potentially promoting tumor progression), in my opinion, their benefit is negligible.
One could argue that these are even detrimental because they have an opportunity cost associated with them. People lose time & money, and believe that they are doing “extremely powerful” anti-aging interventions, which may distract them from other more conducive health-promoting endeavors.
I am unsure whether their promotion is due to ignorance & incompetence or whether their promoters knowingly commit scientifically corroborated fraud for personal financial gain.

Sources & further information
- Podcast: Peter Attia & Richard Miller: The gold standard for testing longevity drugs: the Interventions Testing Program
- Scientific article: Aging and Age-related Diseases: From Mechanisms to Therapeutic Strategies
- Scientific article: Principles of the Molecular and Cellular Mechanisms of Aging
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Part VI: The Exciting Future of Longevity Medicine
When I was 12 years old, my first PC had 1.5 GB of hard drive storage and I was desperately trying to free up some space to install an old-school, CD-ROM-based, 256-bit, Robin Hood video game, which required around 700MB of storage. Now, 15 years later, tiny solid-state flash drives feature up to 256 GB.
When my dad was a child, mobile phones had not yet been invented and TVs were still black and white. When my dad´s dad was a child, any of this would have seemed like an alien invasion. In contrast, when my child has a child, perhaps she will be living most of her life in a VR-based environment with extensive brain-computer interface integration and the real world might be boring (or uninhabitable).

All of this occurred within only a single century, 0.1% of the history of modern humanity, and 1/38.000.000 in the history of life on Earth. Because we are within a moving system, we do not perceive its rate of movement.
Even though there is a vast amount of progress in anti-aging research, until recently, the general public has not been aware of it. And in the coming years, progress will happen even faster – perhaps so fast that we will not even have the time to be astonished. As futurist Ray Kurzweil puts it: “Live long enough, to live forever.” He is referring to people potentially living long enough to reach “longevity escape velocity”.
What follows is a selection of what I think are the most efficient, effective, and promising approaches to increase human lifespan and health span. None of these are uber-optimistic transhumanist sci-fi stuff. Most of these are already in the testing phase in animals (if not humans) and will possibly be available within a few years, or decades at most.
Using the new technologies described shortly, many humans alive today may be able to achieve a longer lifespan than the oldest human being ever recorded (122y) – barring existential catastrophe. Taking into account the warp speed of technological and scientific progress, it is plausible that the first person living for a thousand years has already been born.
Stem cells
The idea of stem-cell-based intervention has been around for quite a long time. Unfortunately, ethical and legal restrictions have ever since hampered progress in this area. Encouragingly, several potential therapeutic approaches are currently being tested in many laboratories around the world.

The basic idea is to get undifferentiated cells to differentiate into whatever cells we want. This is done by using cocktails of cell-type-specific growth factors to guide cellular differentiation. From replacing cardiac muscle after a heart attack to repopulating degenerated dopaminergic neurons in Parkinson’s disease, the possibilities are endless.
Furthermore, next to being used therapeutically (e.g., to repair organ damage), stem cells can be used to increase the number and size of already existing local stem-cell niches, which can repopulate tissues during repair and maintenance.
The currently tested approaches range from stem cell infusions to implantation of stem cells into an existing tissue structure to induction of residual stem cells already found throughout the body. Time will tell whether stem cells are hype or hope.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Tissue engineering
At the moment, only a limited number of (partial) organ replacement therapies are available, including cochlear implants, knee & hip replacement, and heart valves.
In the near future, various approaches to tissue engineering may be used to replace or restore various damaged tissues, organs, or body parts. For example, let’s create a liver. There are multiple ways to do so:
- We could bombard cultured stem cells with a cocktail of transcription factors that nudge the cells towards differentiating into hepatocytes (the main cell type in liver parenchyma). These can then be implanted into an external synthetic tissue scaffold, which then guides organ structure formation. In this way, one can create a liver ex vivo (outside the body), which could then be transplanted into a human.
- We could gene-edit pigs or other GMO mammals in a way that their cells are not immunogenic to human immune surveillance. We could then harvest the liver and transplant it into a human.
- We could resect the most part of a human liver. We could then use cocktails of liver-specific transcription factors to induce remaining local stem cell niches (or autologous grafted stem cells) to recapitulate the program of morphogenesis creating a new liver in situ.
- We could “print” a human liver using biocompatible materials. This can even be done using cell-based “bio-inks” (basically “liver-tissue-paste”).
Eventually, due to advances in tissue engineering, most of the human body could in theory be replaced – other than the brain, as the structural and especially functional connectivity of CNS neurons is (likely) impossible to recapitulate.
Resetting the methylation clock (“partial reprogramming”)
As animals age, they accumulate methylations to their DNA in places where they are not supposed to be. This can be measured in the form of the famous (or infamous) “epigenetic clock” (Horvath clock). Some researchers believe that the progressive accumulation of stochastic methylations gives rise to “noisy” gene expression, meaning that cells do not transcribe the right genes at the right times.
However, no one knows whether these changes to the epigenome are causally related to the aging process, or whether they are just a coincidental bystander feature.
“Epigenetic reprogramming” means resetting the transcription status of a cell to a stem cell level (i.e., to an undifferentiated state). By “partially reprogramming” a cell, one can therefore turn back the epigenetic clock, which is thought to be one (key?) aspect of aging.
Using a combination of three to four master transcription factors (the so-called “Yamanaka factors” – OCT4, SOX2, KLF4, MYC) the epigenomic state of a cell can be reset to a more embryonic-like state with much less epigenetic “noise”. This is usually accomplished via gene therapies (e.g., via viral-based vectors) using doxycycline-inducible promoters.
Reprogramming resembles the process of fertilization, during which the epigenome of the parent cells is effectively reset.
These induced pluripotent stem cells derived from aged cells show extended telomeres and improved nuclear and mitochondrial morphology. Furthermore, their transcriptomic profiles seem to be largely restored. Interestingly, after re-differentiation into other cells, many of these beneficial changes tend to remain in the rejuvenated state.
Some people, such as Sam Altman or David Sinclair, are very excited about this process. However, in my opinion, partial reprogramming is mostly useful on a tissue-level scale, particularly suitable for tissues with little cell growth (e.g., spinal cord, inner ear, eye), but not so much on a whole-body scale.
Speculatively, resetting the methylation clock on a body-wide scale may increase cancer risk. Furthermore, it may cause the partial loss of cellular identity possibly leading to noisy cellular behavior and organismal dysfunction.
In sum, partial reprogramming is an exciting possibility but whether restoring a youthful epigenome translates into a youthful phenotype, and therefore holds the key to a prolonged rejuvenated state, is currently not known. However, there are a bunch of laboratories that are currently working on answering this question.
Of note, instead of whole-body reprogramming, a reasonable and probably safe starting point is to partially reprogram lymphocytes ex vivo (i.e., in the laboratory) and then infuse them back into the patient. Having more youthful and “sharper” lymphocytes, which can easily be killed if something goes wrong, may reduce the risk of cancer (due to stronger immune surveillance), age-associated low-level inflammation (“inflammaging”), and infections.
Gene therapy
Longevity is strongly influenced by genetic factors (much more than most people appreciate). In fact, almost anybody who is a centenarian today is a centenarian because of their exceptional genes – having a healthy lifestyle is helpful but is far from sufficient by itself.
Some of these genes have been identified. There are hundreds of them, some more important than others. By using gene therapy approaches it is possible to either introduce mRNAs for direct protein expression (e.g., by using liposomes), DNA to be shuttled to the nucleus for transcription to mRNA (e.g., by using viral vectors), or even modify our genome (e.g., by using CRISPR-based technologies).
For example, we could:
- Edit, repair, or replace deleterious alleles (e.g., APOE4, BCRA1, APP, etc.)
- Upregulate the expression of longevity genes by modifying the promoter regions or regulating splicing sites (e.g., FOXO3, p53, antioxidant systems, DNA-repair proteins, chaperones, heat shock proteins, autophagy-associated proteins, etc.)
- Downregulate the expression of pro-aging genes (e.g., telomere eroding factors, mTOR and associated proteins, growth factors and proteins involved in their signaling cascade, proteins associated with the PI3K-RAS-MAPK-pathway, senescence-specific proteins, pro-cancer genes, methylation enzymes, etc.)
- Knock in completely new genes (e.g., longevity genes from other species given that they are non-immunogenic in H. sapiens)
Telomerase induction
One gene that deserves special mention is telomerase. Telomerase is an enzyme that lengthens telomeres. With every cellular replication, telomeres progressively shorten. Sufficient telomere erosion is one of the many ways to induce cellular senescence, preventing cells from dividing any further.
In human fibroblasts, this limit is capped at about 75 cell divisions (“Hayflick limit”). Interestingly, the Hayflick limit of mice fibroblasts is about 15, whereas some long-lived giant tortoises have Hayflick limits of about 150 cell divisions.
The Hayflick limit is partially dependent on telomere length, which is partially dependent on telomerase activity. Telomerase is highly active in developing cells and stem cells, allowing them to proliferate without losing the ability to do so (potentially indefinitely).
However, telomerase is a double-edged sword. The more often a cell has divided, the higher the mutation load because at every cellular division, there are going to be a couple of stochastic replication errors. Preventing these cells from dividing prevents potentially cancerous cells from spreading.
Therefore, increasing telomerase activity may help carcinogenesis. On the one hand, telomere erosion (due to excessive cellular replication + lack of sufficient telomerase activity), stops cells from dividing any further, inducing cellular senescence (why this is “bad” is discussed at length here).
Telomerase activation via pharmaceutical drugs or overexpression via gene therapy could be a way to prevent this telomere-erosion-induced pathway to senescence (there are other pathways to senescence also). The downside is that it comes with a potentially increased risk of cancer. However, via advances in cancer screening technologies (especially liquid biopsies -discussed shortly), potential cancers may be found and taken out early.
Parabiosis
Whenever the circulatory systems of a young and an old animal are joined (parabiosis), some rejuvenation happens in the old animal (including the extension of lifespan). This is the reason behind Bryan Johnson using his son as a “blood boy”.
This could be due to either stem cells from the young animal repopulating the more degenerate tissue of the old animal, or it could be due to the transferring of some as of yet mostly unidentified blood-borne factors (e.g., specific growth factors). In the latter case, we could identify these factors, clone them, and use them therapeutically the way we use insulin or growth hormone.
Senolytics
Senescent cells, as holds for every other cell, are kept alive by the continuous signaling of certain growth factors. However, depending on the tissue and cell type, these growth factors (and their receptors) differ. By antagonizing (blocking) the growth factors that are important for the survival of a specific type of senescent cells, some of these “zombie cells” will undergo apoptosis (controlled cell death) much earlier than they would otherwise.
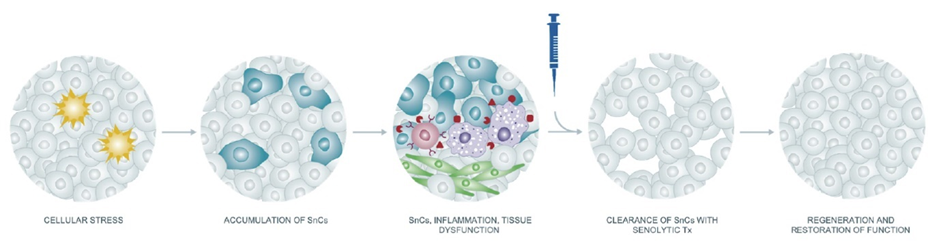
For example, the senolytic agent dasatinib (I discuss my experience with it here) targets certain receptor tyrosine kinases more than others and is therefore rather “tissue-selective” in its senolytic action (e.g., it kills senescent cells in adipose tissue but not heart tissue).
There are multiple other senolytic agents currently in pharmaceutical pipelines, most of them originally developed as cancer drugs. By combining a variety of different senolytics in specific combinations, one could repeatedly clear specific tissues (and perhaps even the whole body) of senescent cells.
After a short course of “hit-and-run” treatment (e.g., for a couple of days), this could be followed by a short course of growth hormone or IGF-1 treatment in order to induce local stem cells to proliferate and to repopulate the now vacant cellular niches.
Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
New milestones in treating chronic diseases
Living longer largely comes down to how long one can delay the onset of chronic disease. Centenarians don’t seem to live longer once they get a disease, they just seem to take longer to get a disease in the first place. As Dr. Peter Attia says: “The superpower of centenarians is how long they can go before they get the first chink in their armor.”
Three chronic diseases together kill around 80% of people in the civilized world. Thus, any advancement made towards preventing or treating these will lift a major limit on human lifespan in an analogous way that antibiotics or vaccines once did.
Because these “diseases” are highly polyfactorial, there are hundreds of conceivable strategies that could be useful for improving their detection, prevention, or treatment. I will now highlight some avenues I find particularly promising.

Atherosclerosis
Cardiovascular disease starts early. Putting people on statins when they are 50 is probably not the best way we should go about it. There are many potential ways in which R&D could help. For example:
- Researching therapies that change the way lipoproteins interface with the endothelium
- Using nanotechnology to remove atherosclerotic plaques
- Identifying genetic or endocrine factors of individuals who never get a heart attack despite the presence of strong risk factors, and then targeting these pathways
I discuss the strategies I personally employ to fight atherosclerosis in more detail here.
Neurodegeneration
To some extent, neurodegeneration happens to everyone. However, there are a vast number of things that could potentially be done to prevent or delay it. For example:
- Researching whether mTOR inhibitors (such as rapamycin) help to prevent or at least delay neurodegenerative disorders
- Genetically modifying plants (e.g., corn, soy, sunflower) in a way that they replace 18-C-omega-6-fatty acids with ALA or EPA omega-3 fatty acids
- Treating prediabetics early and aggressively
I discuss my own anti-dementia protocol in more detail here.
Cancer
There are many things that could help us to better prevent, detect, and fight cancers. For example:
- Researching and developing liquid biopsies and employing them on a population-wide level
- Researching and developing immunotherapy to attack cancers based on individual mutations
- Employing more multi-faceted cancer treatment including immune-based therapies and metabolic therapies (in addition to current treatments)
- More widely employing general anti-aging drugs that are known to reduce the incidence of cancer (e.g., SGLT-2-inhibitors, mTOR-inhibitors)
- Counteracting parts of age-related immunosenescence by vaccinating all of humanity against CMV and EBV (two herpes viruses)
Because cancer will presumably be the final enemy, I discuss exciting developments in cancer detection and treatment in more detail here: Exciting New Avenues to Revolutionize Cancer Treatment.
Nanotechnology
There is a lot of hype around nanotechnological innovations for medical purposes. Theoretically, products of nanotechnology might be used for waste removal (e.g., metals, plaques, metabolic aggregates), as antiviral “robots”, or for carrying out specific functions at a tissue level.
In my non-expert opinion, despite Moore’s law, there is no nanotechnology close to being ready within the next 10-20 years, and true medical nanotech will probably remain sci-fi for a long time.

Furthermore, even with the most incredible future nanotechnologies, in my opinion, it is utopian that our cellular repair and housekeeping machinery will ever be perfect. For example, it will be impossible to completely prevent spontaneous mutations from happening.
Mind uploading
The more I learned about neuroscience, the more I realized that “mind-uploading” is bullshit propagated by tech people without any understanding of biology. Proponents believe that neurons can be replaced with simple on-off nodes in a neural net by making these nodes highly dynamic and capable of adapting and rewiring.
However, neurons are living cells influenced by paracrine & autocrine & endocrine factors in addition to the local non-neural environment (e.g., glial cells, electrolytes, gases). Furthermore, every single neuron is its own cellular micro-universe. All of this makes neuronal activity infinitely complex and not just a product of summing up various inputs to produce an output.
Some people want to go around this problem by crudely simulating neurons. However, approximating real-life neuronal activity would presumably entail simulating the stochastic and probabilistic nature of quantum mechanics at least to some extent.
Furthermore, even if one could do all that, the activity of our nervous system depends on non-stop input from sensory cells transducing the external and internal environment. Everything my nervous system does at any given point depends to a large degree on this continuous input (next to some spontaneous neural activity).
If one were to perfectly simulate the nervous system, where would this continuous sensory input come from?
This sensory input does not just depend on our “five” senses, but dozens of sensory modalities and thousands of sensory feedback loops that never even make their way up to the neocortex and thus into consciousness.
For example, the millions of chemo- and mechanoreceptors all over the body continuously transmit data to the brain stem, which in turn modulates CNS activity in a specific way and imperceptibly guides my thinking, feeling, and acting. In my opinion, both problems (simulating neurons; simulating input) are insurmountable. Not just technically.

Off-topic note on non-biological consciousness
While mind-uploading is, in my opinion, utopian, it is indeed possible that our technological children will survive and have their own “minds”.
It is more likely than not that the right kinds of computations are sufficient for the emergence of consciousness. Given that “substrate independence” is true, consciousness is just an emergent property of complexly arranged matter (e.g., as in our brains).
Consequently, when implemented with sufficient complexity, the “right” processing architecture might cause consciousness to arise spontaneously. If this architecture were modeled after the human brain, it would probably be characterized by distributed signal representation, large-scale signal integration, and swift feedback and feedforward loops.
Given that, in theory, this is not limited to biological systems, consciousness might spontaneously emerge as a byproduct in artificial agents (including software) that have a suitable (neuro-inspired?) architectural complexity (or anything functionally equivalent) – possibly including noise-driven assemblies resembling spontaneous cortical activity.
Sources & further information
- Scientific article: Stem cells: past, present, and future
- Scientific article: The Effects of Parabiosis on Aging and Age-Related Diseases
- Scientific article: In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming
Conclusion
Everywhere on Earth, infectious diseases and limited resources have restricted population growth and limited human lifespan ever since. Starting around 100 years ago, humans began to eliminate these lifespan shackles via improvements in food production, hygiene, sanitation, and the advent of antibiotics and vaccines.
As a result, over the course of only a single century, the human average lifespan more than doubled and progressively approached maximum biological lifespan, which is thought to be around 100-130 years. Now, there are few external lifespan-limiting factors left to eliminate (In my opinion, for most people, exposure to pollution or environmental toxins is not high enough to significantly limit their lifespan.), and nowadays human lifespan is mostly limited by internal factors: aging itself.

Aging is spontaneous and happens to everyone. Therefore, it is considered a natural process and not a disease. “Disease” is defined as “a disorder of structure or function in an organism, especially one that produces specific signs or symptoms and is not simply a direct result of physical injury”. Aging fits this definition perfectly. This meta-disease is chronic, progressive, with a 100% incidence and a 100% lethality rate.
Unfortunately, fighting this disease is hard. There are dozens of different mechanisms working together (and independently) to produce the syndrome we call “aging”. Even if we were to eliminate all but one of these, at least some degeneration of an organism over time would still occur. Therefore, fully “curing” aging is impossible, and everyone who says otherwise either has little idea of what aging is, or is a charlatan, or both.
Furthermore, because some aging mechanisms are irreversible (e.g., spontaneous mutations, waste accumulation), aging can only be slowed but hardly reversed. Hence, fighting aging is mostly about prevention, which also protects against its complications: the huge spectrum of age-related diseases that cause most deaths and great suffering throughout humanity.
Fighting aging requires a complex array of strategies attacking it from many different angles. As explained in Part III, this includes the targeting of the cellular drivers of aging, reducing exogenous drivers, bolstering the repair machinery, and repairing compromised organ systems. This would allow for aging to be delayed until progressive neuronal death, an ever-larger mutation load, or total telomere erosion become the absolute lifespan-limiting factor.
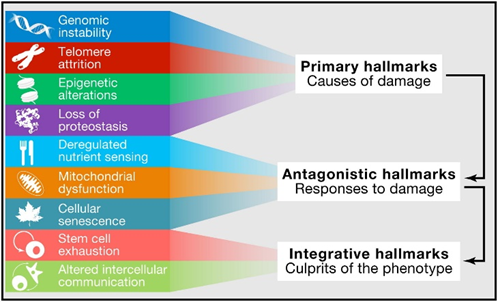
Currently, available interventions such as rapamycin might be the next step-functions in humanity’s quest towards longer and healthier lives, in the same way that vaccines and antibiotics once were, though conclusive data is not in yet.
In the near future, many other potential step-functions might be available to potentially prolong human health span. Examples include telomerase manipulation, methylation-clock resetting, stem cell replacement, tissue engineering to replace aged and dysfunctional organs, and perhaps even various nanotechnologies.
Medicine has perfected the art of prolonging disease, not prolonging health. The field of anti-aging medicine needs more attention and funding. From a purely economic perspective, paying for anti-aging research and longevity interventions would not only save a nation a lot of money (less direct healthcare costs) but also lead to large financial gains (increased productivity and thus a better economy and more taxes).
Decreased health or well-being – no matter the cause – always has negative consequences for fellow humans. For example, if I feel and function badly, I drag down loved ones, I am less productive, I contribute less to the well-being and productivity of others, and I am more likely to lead a bad lifestyle (and thus increase my risk for chronic diseases resulting in decreased future productivity & taxes and increased costs to the healthcare system).

Ethical and demographic implications aside (e.g., two-class society, overpopulation), there are a variety of currently available interventions that could potentially improve health span and lifespan on a population-wide level.
- Changing the “health care” system from a system of “sick care” (people currently only interact with the health care system when there is a problem) to a true system of health care and appropriately aligning incentives. For example, setting up payment in a way that financially incentivizes doctors to keep the patient healthy instead of just prolonging the suffering phase. (This is obviously much easier said than done.)
- Requiring that a reasonable and fixed percentage of a country’s medical budget is spent on public health interventions. Currently, in most industrialized nations, only about 1-3% of the medical budget is spent on prevention.
- Teaching the principles and benefits of exercise & sleep & nutrition & mindfulness in schools.
- Getting rid of food subsidies for corn, soy, etc. that allow food companies to produce dirt-cheap crap.
- Offering free & individualized hormone replacement to everybody beyond a certain age who wants it.
- Making more use of price policy to drive behavioral change, which is arguably the most effective way to change behaviors on a population-wide level. For example, collecting additional taxes on sugar and junk food, which will compensate partly for the associated medical costs that have to be otherwise borne by society. Or raising the price of cigarettes to astronomical amounts, which would get rid of around a third of all cancers, a lot of COPD cases, and a non-trivial percentage of cardiovascular deaths.
- Banning the use of crappy vegetable oils in restaurants and food processing. Alternatively, genetically modifying certain plants (e.g., corn, soy, sunflower) in a way they synthesize greater amounts of monounsaturated fatty acids or omega-3-fatty acids instead of 18-C-omega-6-fatty acids.
- Investing in R&D for a vaccine against viruses of the herpes family, which are associated with all kinds of age-related diseases. This would counteract some of the age-associated immunosenescence and low-level inflammation.
- Radically changing the standard of care for prediabetic and diabetic patients, who comprise about a quarter of the adult population in Western countries. This would include early and aggressive management, changing dietary guidelines for prediabetics from low-fat to low-carb, and delaying the use of insulin.
- Giving out GLP-1 agonists more widely for weight management, which would help with all major chronic diseases (cardiovascular diseases, cancer, dementia). Furthermore, making a cheaply available and non-immunogenic leptin analog may help people lose even more weight, and more importantly, keep it off.
- Researching optimal dosages and regimens for rapamycin use, and if said research turns out favorable, offering it to people above the age of 30.

Carefully selected and employed, large-scale anti-aging interventions would allow millions of people to lead not just longer but also better lives. Regardless of the increase in personal well-being, society would presumably benefit as well.
- This would reduce the direct costs of illness, leaving more funds for other matters.
- Given that an experienced and vital workforce is the cornerstone of a healthy economy, there might be large indirect financial, material, and societal gains stemming from increasing and prolonging the productivity of individuals.
- Individuals would have a longer and larger impact on others by doing whatever they directly or indirectly do to contribute, moving towards a society where old & experienced people are physically and mentally capable of leading companies and countries.
In the future, 80 will feel and look like 50. Is it natural? No, because evolution could not have cared less about humans functioning well after the age of 50. But what is still “natural” in our world anyway?
With the tools already available to us, combined with what is heading our way in the coming decades, the limits may be the stars. Literally.

