If you are just here for the practical aspects, feel free to skip the science sections.
Insulin
Metabolism in vertebrates is primarily regulated by hormones. Of these, insulin is among the most important and can be called the “master control” hormone of metabolism.
Insulin is the “hormone of abundance”, and its job is to store energy. This goes far beyond the cellular uptake of glucose. Insulin stimulates the activity of hundreds of different proteins, and its downstream effects alter the expression of around 2000 genes.
In short, insulin stimulates whole-body anabolism (“growth”), while its absence induces whole-body catabolism. The former of these is readily seen in type II diabetics (who get fat easily) whereas the latter of these is readily seen in type I diabetics (who lose body weight quite easily & fast).
The upsides and downsides of insulin
In biology, things are rarely “bad” or “good”. Insulin is no exception. I personally do not want to be “low insulin all of the time” but rather “low insulin resistance all of the time”. But before we get to that, I will briefly discuss the positive and negative aspects of insulin.

Subscribe to the Desmolysium newsletter and get access to three exclusive articles!
Insulin is the most anabolic hormone in the human body (hence its abuse in bodybuilding) and turns on a variety of growth pathways. While this is good for a developing organism, these growth-promoting effects also accelerate the aging process, which may be partly considered a continuation of the pre-adulthood growth program.
Chronic hyperinsulinemia, as it happens in states of insulin resistance, will cause growth pathways to be always “on”, which promotes a host of age-associated diseases such as cancer, cardiovascular disease, and neurodegeneration.
Furthermore, hyperinsulinemia inhibits lipolysis (the breakdown of triglycerides in fat tissue) and beta-oxidation (the breakdown of fatty acids for energy) while stimulating lipogenesis (the synthesis of fatty acids) and adipogenesis (the storage of fat in adipocytes), thereby promoting fat gain, which wreaks havoc on metabolic health through a variety of interdependent mechanisms. As one researcher put it: “Obese people are essentially starving in an encasement of blubber.”
Unfortunately, insulin is extensively used in the treatment of early-stage type II diabetics to keep plasma levels of glucose within a healthy range. Adding insulin to an early-stage type II diabetic patient may help with micro-vascular disease (which impairs kidney function, eye health, and brain health) but at the expense of accelerating macrovascular disease (atherosclerosis).
Unfortunately, few physicians realize that controlling blood glucose levels is only half of the job, and the other half is keeping area-under-curve insulin levels low, and injecting loads of insulin into an organism that is already teeming with insulin is probably among the worst current practices in medicine.
Despite its bad reputation, insulin serves many vital roles, and most tissues require some of it for proper function. If insulin levels are kept at very low levels for extended periods of time, many detrimental changes occur, including plummeting energy levels, organ shrinkage, and immunosuppression.
Adequate insulin levels are also necessary for vitality. Insulin stimulates energy levels from within multiple points in the central nervous system. It also stimulates cellular energy metabolism (especially glycolysis), increases hepatic and renal conversion of T4 to T3, induces hepatic IGF-1 synthesis, and it stimulates leptin secretion. Moreover, it promotes hormone bioavailability by regulating levels of hormone-binding proteins (e.g., SHBG, CBG, TBG, IGFBP-3).
With very low levels of insulin, all of these processes are impaired, and vitality often takes a hit. I discuss this in more detail in my article on ketogenic diets.

Many tissues highly depend on stimulation by insulin (e.g., kidneys, liver, endocrine organs, etc.), and if insulin levels are kept at near zero for too long and too often, many detrimental changes occur because said tissues are never adequately stimulated to grow and maintain.
Insulin’s effect on energy levels can be readily seen when patients with Type I diabetes are treated with insulin. Within the span of just a few days, their energy levels often increase dramatically.
Of note, people who are overweight in childhood will have a greater beta-cell mass and thus a greater insulin production for life. All else being equal (e.g., regardless of energy expenditure centers in the brain “getting used to” a higher weight/appetite setpoint), if someone is overweight early, they will gain fat more easily forever.
Insulin resistance
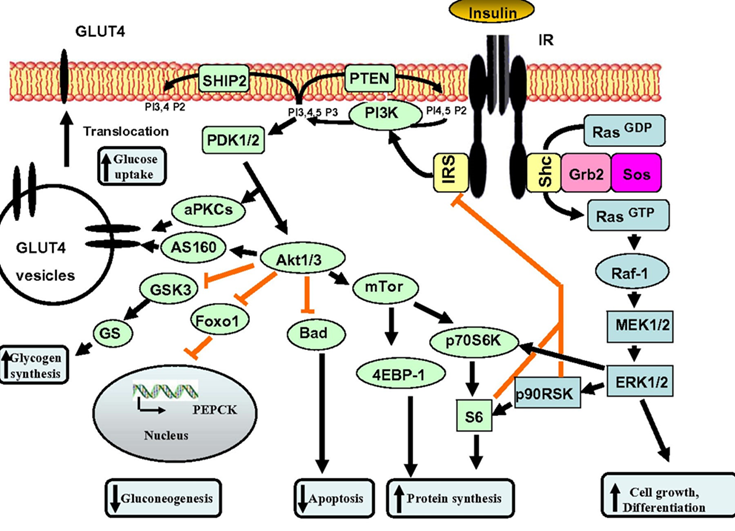
The term “insulin resistance” is very misleading. Cells are not at all resistant to insulin, they are just resistant to the glucose-lowering effects of insulin, which is only one of many effects. Most of the hundreds of other effects of insulin still work normally, including induction of lipogenesis, inhibition of lipolysis and beta-oxidation, and stimulation of mTOR.
Insulin is most famously regulated by blood glucose levels. However, in an insulin-resistant state (or better put: in a state in which insulin is incapable of stimulating insulin-mediated glucose uptake) insulin is not able to stimulate proper glucose uptake from the bloodstream. The result is hyperglycemia with a feed-forward effect on hyperinsulinemia.
This “insulin all of the time” then does a lot of havoc, including:
- Stimulation of de-novo lipogenesis in the liver, which leads to visceral fat and dyslipidemia
- Stimulation of fat storage and prevention of fat breakdown (lipolysis), which leads to a hard time losing fat and/or obesity
- Large-scale alterations in cellular metabolism
- Gene expression changes in an “anti-longevity” way
- Stimulation of growth pathways (mTOR, PI3K-ras-pathways), which promotes cancer, atherosclerosis, and inflammation
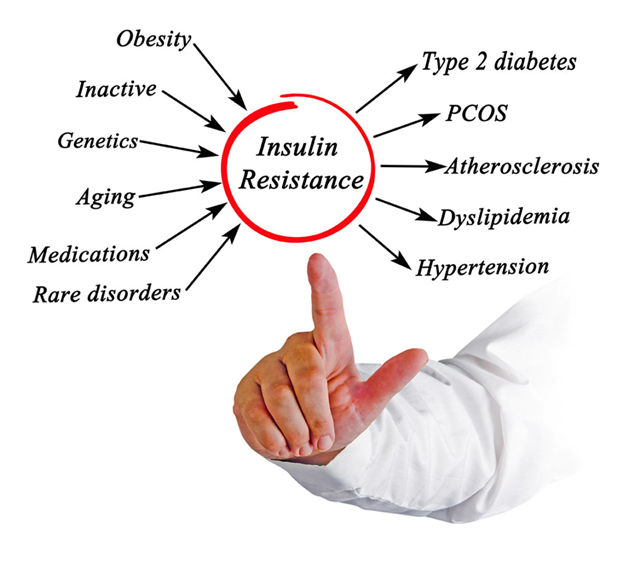
Now the question remains: “I get that insulin resistance is bad, but how does it arise?” There are multiple paths to it, including visceral fat, hypercortisolemia, inflammation, or a systemic elevation of free-fatty acids due to whatever cause.
While these pathways are not mutually exclusive, the “visceral fat”/fatty liver pathway is presumably the most common. I discuss visceral fat in more detail here.
How does insulin resistance arise mechanistically?
Mechanistically, things are quite complex. In short, it is thought that locally elevated levels of free fatty acid (e.g., as it happens around visceral fat), turn on a certain intracellular pathway that phosphorylates the insulin receptor (more technically, a protein complex binding to it named insulin-receptor substrate 1) in a certain way that this insulin receptor becomes “bad” at promoting GLUT4 insertion, thereby leading to a reduced effect on cellular glucose uptake.

This state is (improperly) called “insulin resistance” because fat and muscle cells (the majority of body mass) cease to properly respond to the glucose-uptake-promoting effects of insulin (but they still respond to the other effects of insulin).
The resulting hyperglycemia then causes hyperinsulinemia, which then initiates a vicious cycle. More visceral fat accumulates, which then causes more hyperinsulinemia, which then wreaks a lot of havoc (as explained above) that ends up in metabolic syndrome and often obesity.
Furthermore, a fatty pancreas develops, and the fatty pancreas combined with a high insulin demand, causes burnout of pancreatic beta-cells, which then start to die. After years to decades, insulin secretion starts to decline, and exogenous insulin needs to be used. Now, people are diagnosed with full-blown type II diabetes. Currently, about 15%-20% of the medical budget is spent on treating DM II and its complications.
Evolutionary biology
Some researchers hypothesize that in our evolutionary past, insulin resistance served an important purpose. The elevation of free fatty acids leads to the phosphorylation of the insulin receptor leading to reduced cellular glucose uptake.
Evolutionarily speaking, the only times when levels of free fatty acids were elevated were times when the animal was in starvation. The combination of low insulin, high cortisol, high growth hormone, and high adrenaline (as is the case in starvation) leads to a massive breakdown of fat tissue, resulting in a massive rise in levels of free fatty acids.
In starvation, nutrients need to be strategically allocated to support vital organ function. By inducing insulin resistance in the liver, muscles, and fat tissue, these tissues (which comprise about 80% or so of body mass) stop using glucose for energy, which is then reserved for the brain. Because the glucose uptake by the nervous system is insulin-independent, body-wide insulin resistance comes in handy.

Nowadays, the overconsumption of carbohydrates, lack of activity, visceral fat, and obesity (all of which were mostly absent in our evolutionary past) lead to a state of elevated levels of FFA without starvation, but insulin resistance is induced nonetheless. So, this beneficial evolutionary mechanism has gone awry.
Strategies I use to keep my insulin sensitivity high year-round
Keeping body fat levels low
Visceral fat is, for the most part, a function of body-fat percentage. I aim to keep a low body fat percentage (roughly around 12%) year-round. Once to twice a year, I measure my body fat % via a DEXA scan.
Keeping body fat low improves insulin sensitivity through a variety of mechanisms, and it reduces whole-body levels of inflammation. Keeping insulin sensitivity high and reducing levels of inflammation are both essential for good metabolic health.
Tools I use for keeping body fat low include exercise, TRT lite, and a very low dose of metreleptin. The addition of a low dose of metreleptin allows me to keep a low body-fat percentage without suffering from the starvation-related decrease in hormones, energy, and mood. I eat about 3000-3500 kcal per day. In the past, I have used semaglutide for 3-4 years though I came off because eating felt like a chore.
I also restrict sugar consumption, which specifically induces the accumulation of visceral fat.
Visceral adiposity leads to a fatty liver, which wreaks havoc on all aspects of metabolic health, particularly insulin sensitivity. The science is quite complex and has to do with local adipokine signaling, the local elevation of free fatty acids activating the inflammasome, and free fatty acids inducing insulin resistance.
I discuss the importance of visceral fat in more detail here: The Six Pillars of Metabolic Health
Keeping my body fat levels low is perhaps the most important point on this list.
Avoiding large glycemic loads
I (try to) avoid eating a lot of high-glycemic carbohydrates (“bad carbs”) in one sitting, particularly if I have not exercised beforehand. I discuss my diet in more detail here.

In the past, I tried to “hack” this by using acarbose or SGLT-2 inhibitors, both of which reduce post-meal glucose excursions. However, acarbose consistently spiked my liver enzymes and SGLT-2 inhibitors consistently dehydrated me. Needless to say, I stopped taking both. I discuss my experience with metabolic drugs in more detail here.
Optimizing sleep
There are multiple ways in which bad sleep hampers metabolic health.
- In a sleep-deprived state, cortisol levels are generally high. Cortisol has a huge effect on various aspects of metabolism. In Cushing syndrome (excess cortisol levels) metabolic health is usually crap.
- Good sleep is necessary for proper growth hormone release. Growth hormone is anabolic to muscle and catabolic to fat, particularly visceral fat..
- The nervous system itself is quite a powerful regulator of metabolic status. For example, through vagal nerve endings, the nervous system affects liver metabolism and insulin secretion. Bad sleep is usually associated with greater basal insulin secretion, presumably mediated by vagal control of the pancreas.
- In a sleep-deprived state, willpower and metacognition are poor, which leads to bad food choices and forgoing exercise, both of which are causally related to metabolic health.

I discuss how I optimize my sleep in more detail here.
Maintaining a decent amount of muscle mass

Having a decent amount of muscle mass is known to improve pretty much every aspect of metabolic health, including insulin sensitivity, nutrient partitioning, and metabolic flexibility. Muscle is a very insulin-sensitive tissue that will function as a glucose sink, particularly post-exercise. Therefore, all else being equal, more muscle equals greater insulin sensitivity.
Muscle mass is acquired by progressive overload and an adequate caloric intake. I discuss my approach to building and maintaining muscle mass in more detail here.
Endurance exercise
Once or twice per week, I do a 1h session of zone II cardio. Below is a screenshot from my Polar data of what a zone II session looks like for me (I usually end with a 5-minute high-intensity part). My average heart rate is between 70-80% of my maximum heart rate (which is a little over 190 bpm).

A proper endurance exercise session is known to improve insulin sensitivity for at least 24 hours. I am always amazed at how potent this is when I look at my CGM data.
Exercise, in particular endurance exercise, leads to countless adaptations in skeletal muscle. One of the presumably most important changes is an improvement in mitochondrial function and a stimulation of mitochondrial biogenesis. Said another way, endurance exercise increases both the number as well as the quality of mitochondria, which has a variety of positive effects on metabolic health.
Furthermore, endurance exercise stimulates an insulin-independent GLUT-4 translocation, which will reduce blood glucose levels in an insulin-independent way.
Moreover, endurance exercise leads to a depletion of intracellular nutrient stores (particularly glycogen), which activates a metabolic master switch enzyme called AMPK, with downstream effects of increasing insulin sensitivity.
On an epigenetic level, endurance exercise also leads to altered expression of a large number of genes.
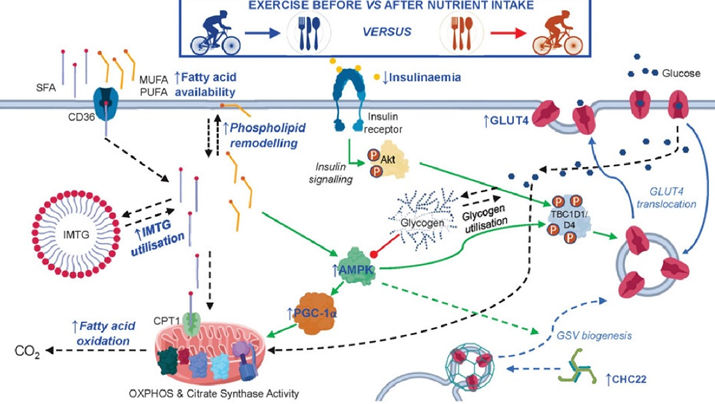
Because of these changes, a single endurance exercise session is thought to increase insulin sensitivity for the next 24 hours or so. By regularly depleting glycogen stores, dietary glucose is handled the way it should be, namely, by being funneled into hepatic or muscle glycogen synthesis. Conversely, if glycogen stores are always tapped out, excess glucose is biochemically much harder to manage and comes with a host of unfavorable biochemical alterations.
As always in biology, many processes are happening in parallel and a plethora of changes are cross-interacting, influencing, and driving each other. Parallelism is something our brains are just awfully bad at grasping because they evolved to cognize linearly in space and time.
In sum, the end result is that endurance exercise has an incredible effect on insulin sensitivity and mitochondrial function, both of which are at the center of metabolic health.
Optimizing hormones
I watch a number of hormones closely, including sex hormones, thyroid hormones, IGF-1, and leptin. Having “youthful” levels of these hormones will improve nutrient partitioning, insulin sensitivity, muscle growth, body fat levels, and hundreds of other things.
Furthermore, hormones have powerful effects on energy levels, mood, vigor, and vitality, and therefore, they make leading a healthy lifestyle much easier.
Currently, I only supplement with HCG and a low dose of metreleptin.
Metabolic drugs
I take weekly rapamycin and allopurinol. I discuss my experience with these drugs in more detail here: Metabolic Drugs
Supplements
I am quite sure, that, compared to the other things on this list, supplements do quite little. I take most of them with a “just-in-case” mentality, and I am equally uncomfortable taking them compared to not taking them. Nonetheless, I have been taking a variety of supplements for about 4-5 years, and nothing “bad” has happened.
I discuss the supplements I take, and why I take them, in more detail here.
Other tactics aimed at improving metabolic health
The strategies discussed above are a selection of the tactics I follow to maintain good metabolic health. The full list of tactics, some of which are not particularly aimed at specifically increasing insulin sensitivity and therefore were not included in this article, can be found here.
Bonus – My low-dose insulin experiment
My life is a biological laboratory and I am constantly running experiments, some of them “work” but most of them fail. However, almost every experiment teaches me something.
For years, I kept my insulin sensitivity very. For example, when I eat a candy bar (for science), my blood glucose is back to baseline, and often even below baseline, after already 30 minutes. I attribute this to my low levels of body fat, strategic exercise, and metabolic hacks, including GLP-1 and leptin manipulation.
However, a consequence of high insulin sensitivity is low basal levels of insulin. Because little insulin is required to maintain blood sugar at a healthy level, little is secreted by the pancreas.
Despite of what all the “influencers” in the sphere are preaching, insulin is not exclusively bad. Sure, it drives the mTOR pathway (and therefore aging) and hyperinsulinemia is associated with all kinds of chronic diseases, such as cancer, dementia, and atherosclerosis.
But as so often, the dose makes the poison. Insulin is similar to insulin-like-growth factor 1 (IGF-1). Both of these are anabolic hormones, perhaps the most anabolic hormones known to mankind.
Insulin is also responsible for driving glucose and amino acids into cells, inducing them to be active. Furthermore, as a medium-term satiety peptide, it gives a “go-ahead” signal to the hypothalamus that the organism has plenty of resources to use for activities besides acquiring food.
When “the hormone of abundance” is very low, such as in type I diabetes, individuals basically “wither away”, both mentally and physically – in part because their cells stop metabolizing nutrients and enter a kind of “conserve resources mode”.
A normal physiological production of insulin is about 60 IU per day, 30 IU as a basal insulin (constantly trickled into the blood stream) and roughly 30 IU in response to eating food (prandial production of insulin).
So, what would happen if I, say, add 5 IU of a long-acting insulin to my physiology? Presumably not much because that would only slightly elevate my total insulin. Wrong. Even such a low amount of insulin significantly increased my energy levels – particularly on day 1 and day 2. And the change in how I felt was quite striking. Furthermore, my heart rate variability (as measured by my Oura ring) decreased by about 10 points and my resting heart rate at night increased by 5 points (from about 45bpm to about 50bpm).
All of these changes are indicators that my sympathetic nervous system activity increased. After 10 days, I then stopped the insulin injections, and on that day I felt tired, “weak” and moving around was particularly onerous. The changes in resting heart rate and HRV reversed – indicating that my sympathetic nervous system output had dropped again.
I also got lightheaded multiple times, presumably because my CNS had partially adapted to the higher SNS-activity, analogous to how people feel tired and weak after having taken simulants for a couple of days and then suddenly stopping.
I then reintroduced 5 IU of insulin degludec, and lo and behold, my SNS activity rose again.
Lessons #1: My basal insulin levels are very low – perhaps too low.
Lesson #2: While having very low levels of insulin may be good for longevity, it may not be optimal for vitality. As so often, vitality and longevity are at odds.
Lesson #3: Insulin degludec had more pronounced effects that insulin glargine or insulin detemir – 3 different forms of long-acting insulins that differ in their half-lives and presumably also in their ability to cross the blood brain barrier.
Sources & further information
- Podcast: Peter Attia & Richard Miller: The gold standard for testing longevity drugs: the Interventions Testing Program
- Podcast: Peter Attia & Gerald Shulman: A masterclass on insulin resistance—molecular mechanisms and clinical implications
- Scientific article: Role of Insulin in Health and Disease: An Update
Disclaimer
The content available on this website is based on the author’s individual research, opinions, and personal experiences. It is intended solely for informational and entertainment purposes and does not constitute medical advice. The author does not endorse the use of supplements, pharmaceutical drugs, or hormones without the direct oversight of a qualified physician. People should never disregard professional medical advice or delay in seeking it because of something they have read on the internet.
